Deposition Date
2018-03-21
Release Date
2018-08-15
Last Version Date
2024-01-17
Entry Detail
PDB ID:
6G1I
Keywords:
Title:
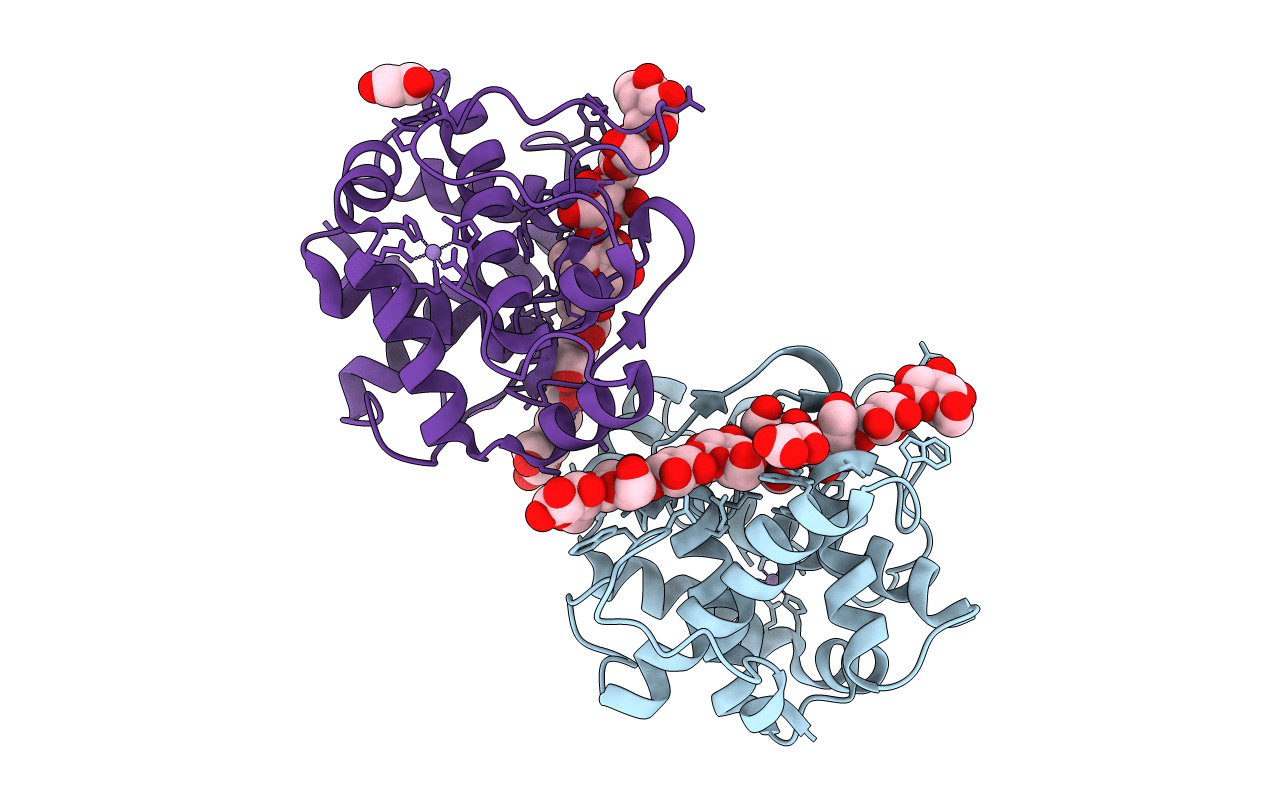
GH124 cellulase from Ruminiclostridium thermocellum in complex with Mn and fructosylated cellopentaose
Biological Source:
Source Organism:
Host Organism:
Method Details:
Experimental Method:
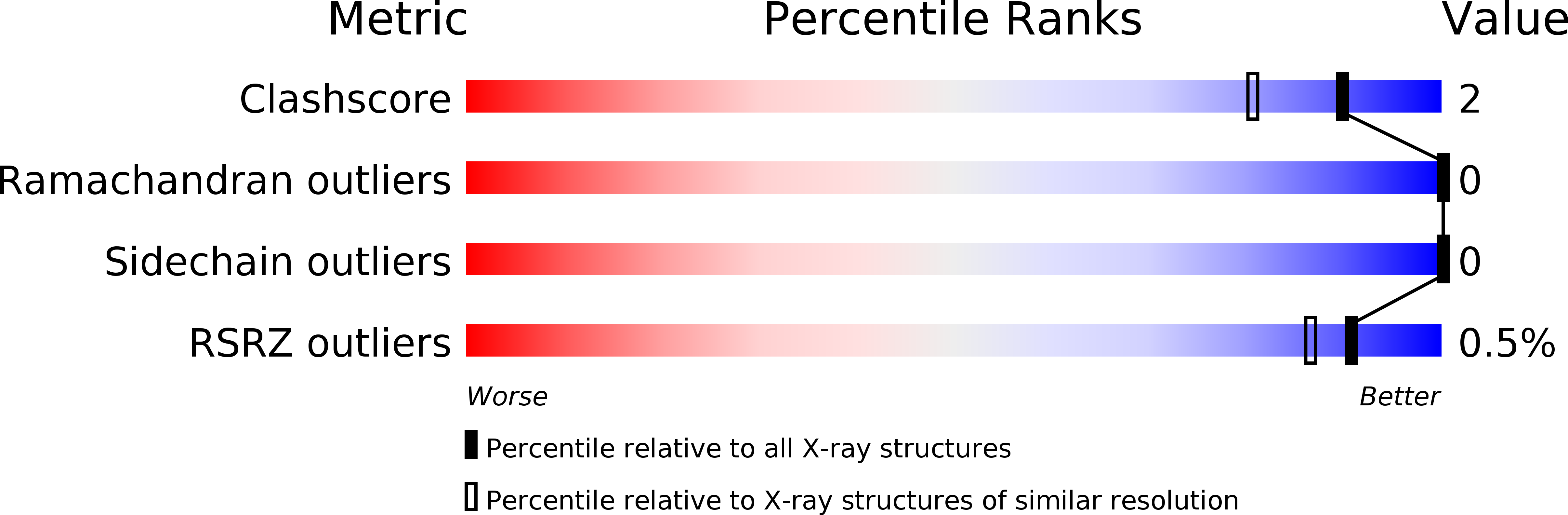
Resolution:
0.99 Å
R-Value Free:
0.14
R-Value Work:
0.13
R-Value Observed:
0.13
Space Group:
P 21 21 21