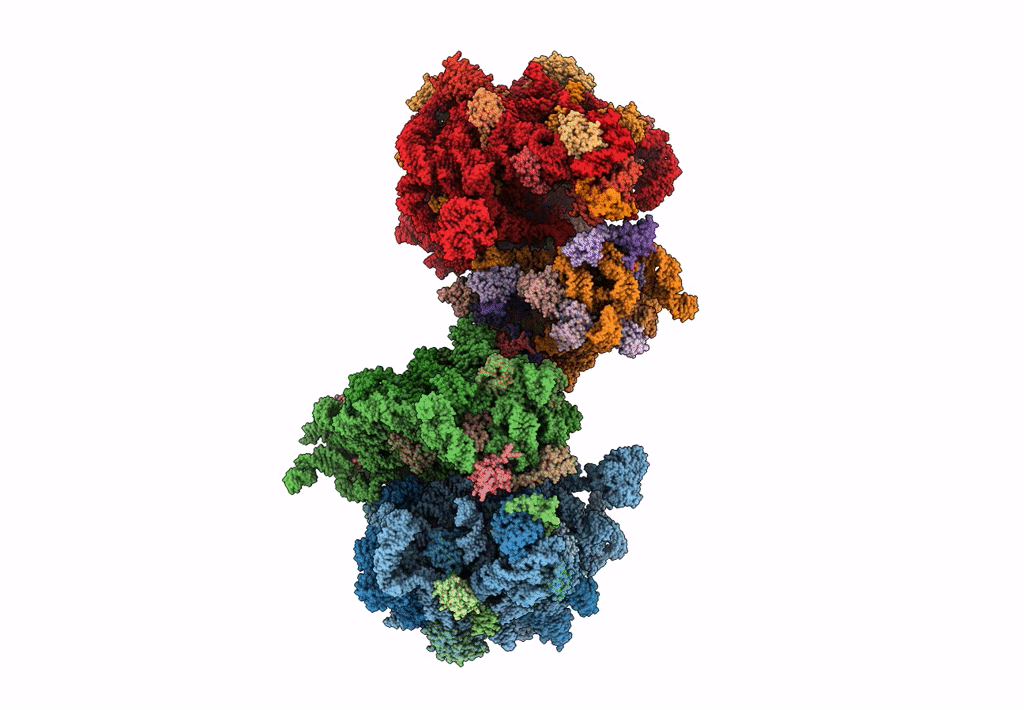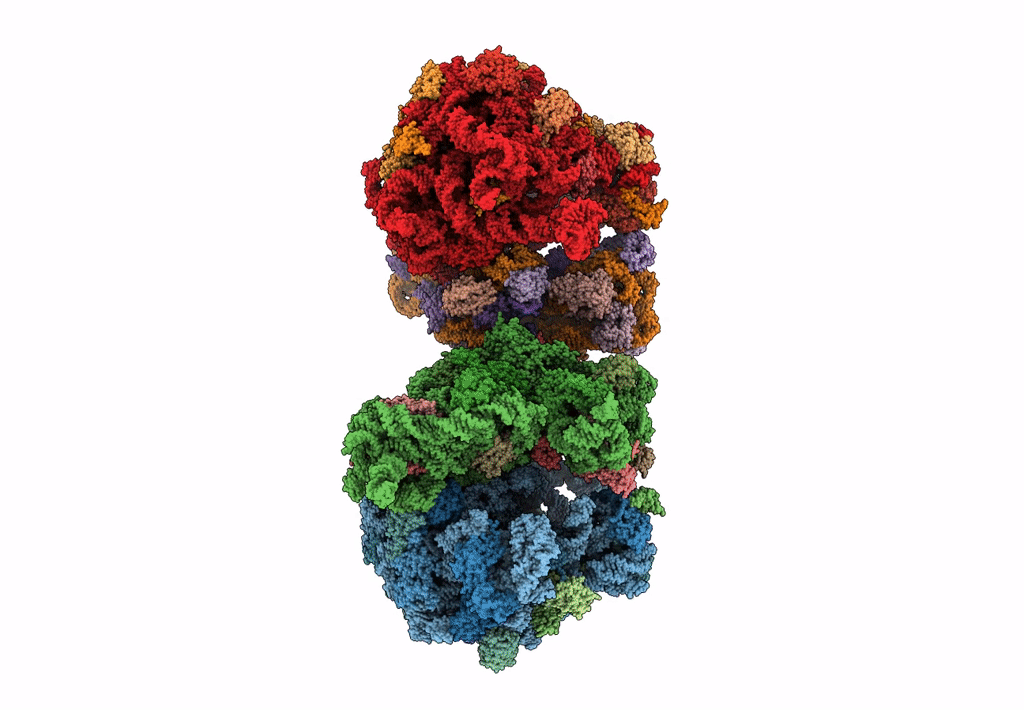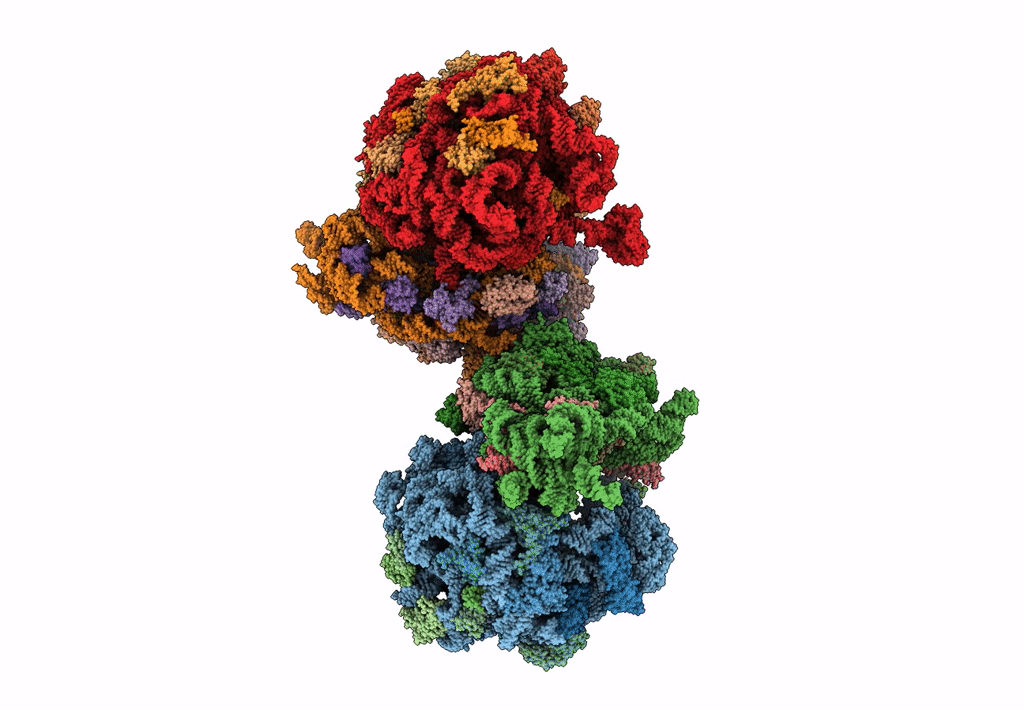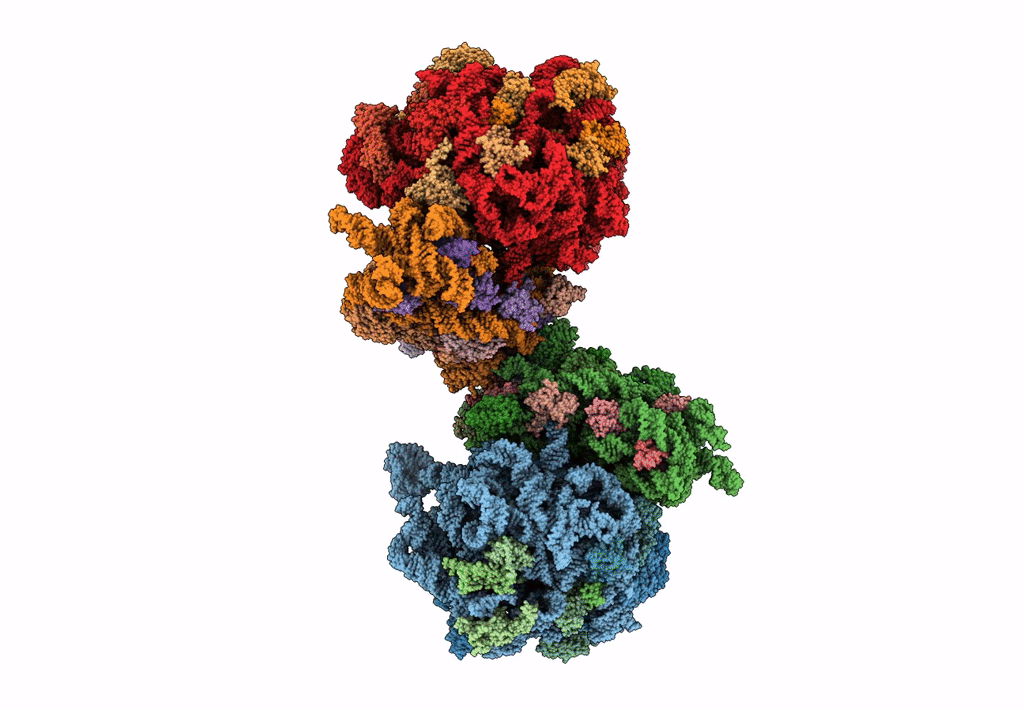
Deposition Date
2018-03-08
Release Date
2018-03-21
Last Version Date
2024-11-13
Entry Detail
PDB ID:
6FXC
Keywords:
Title:
The cryo-EM structure of hibernating 100S ribosome dimer from pathogenic Staphylococcus aureus
Biological Source:
Source Organism(s):
Staphylococcus aureus (Taxon ID: 1280)
Staphylococcus aureus (strain bovine RF122 / ET3-1) (Taxon ID: 273036)
Staphylococcus aureus (strain bovine RF122 / ET3-1) (Taxon ID: 273036)
Method Details:
Experimental Method:
Resolution:
6.76 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


