Deposition Date
2018-02-28
Release Date
2019-03-20
Last Version Date
2024-11-13
Entry Detail
PDB ID:
6FV2
Keywords:
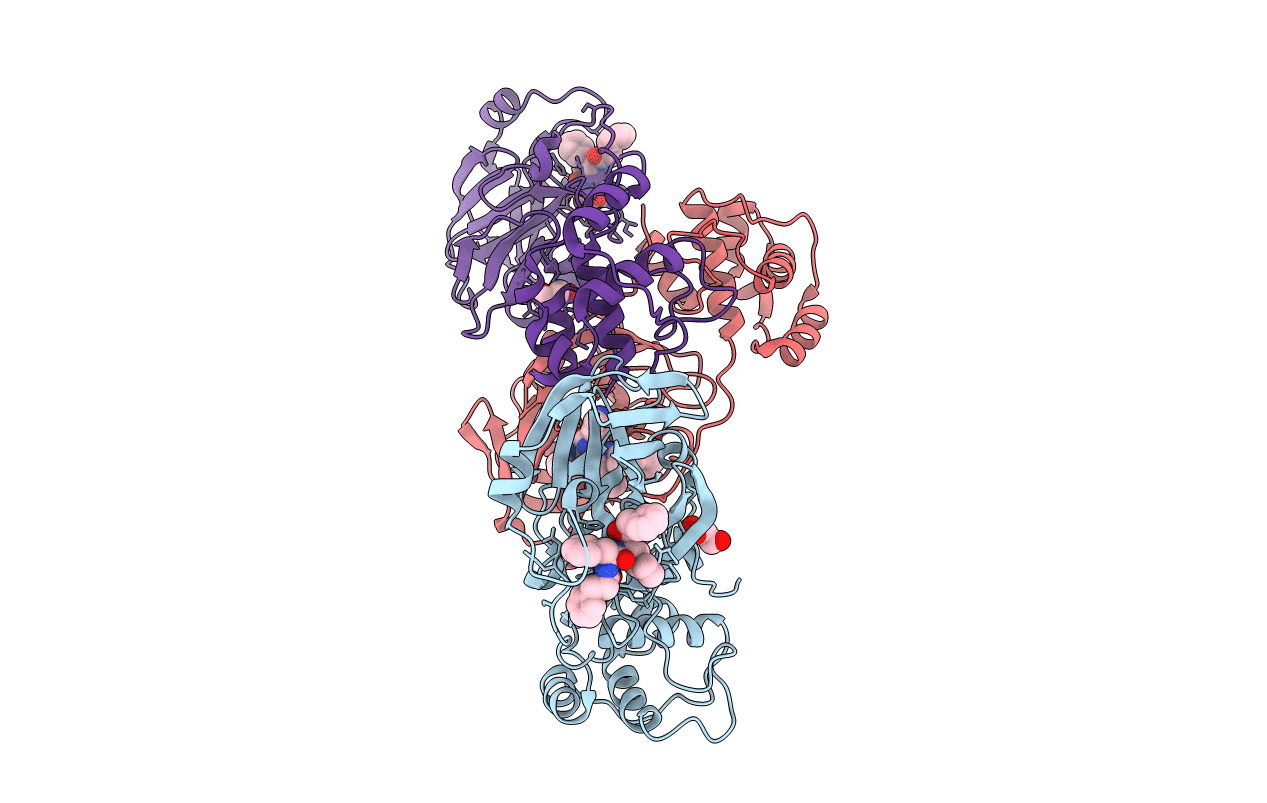
Title:
Structure of human coronavirus NL63 main protease in complex with the alpha-ketoamide (S)-N-benzyl-3-((S)-2-cinnamamido-3-phenylpropanamido)-2-oxo-4-((S)-2-oxopyrrolidin-3-yl)butanamide (cinnamoyl-phenylalanine-GlnLactam-CO-CO-NH-benzyl)
Biological Source:
Source Organism(s):
Human coronavirus NL63 (Taxon ID: 277944)
Expression System(s):
Method Details:
Experimental Method:
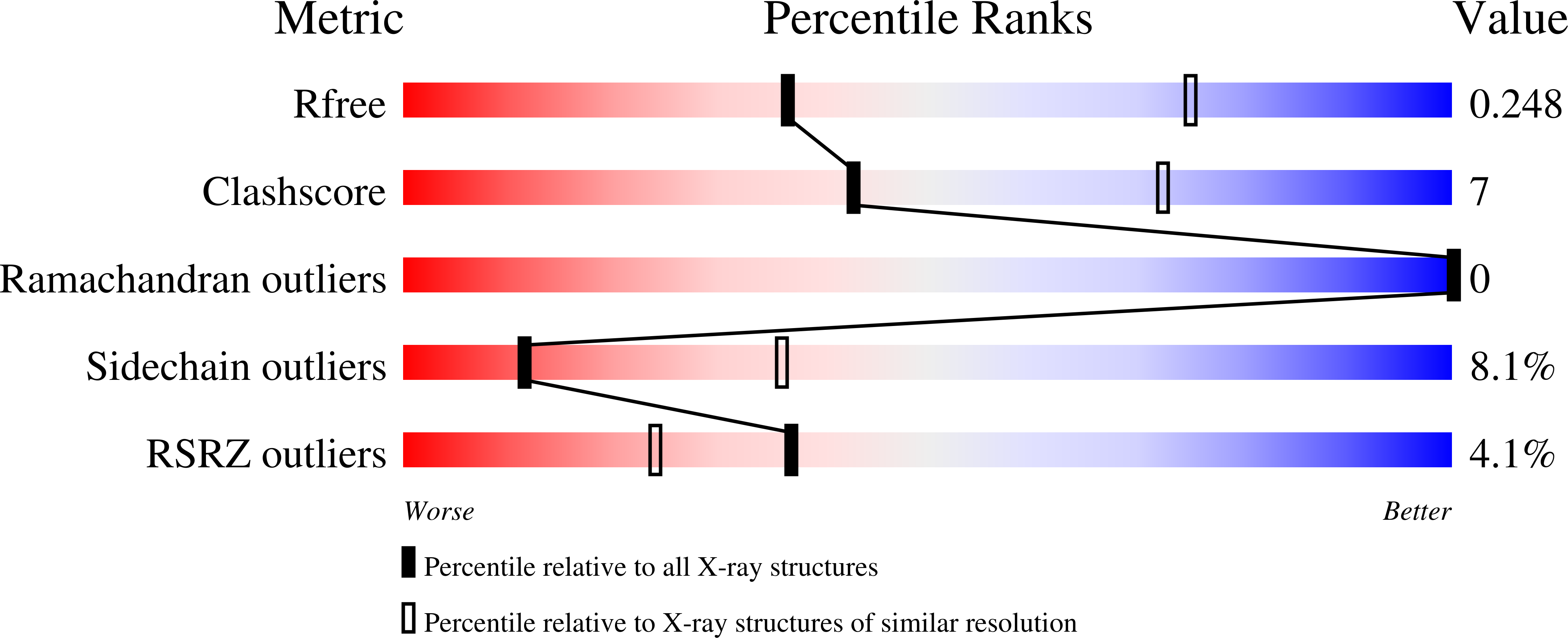
Resolution:
2.95 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
C 2 2 21