Deposition Date
2018-01-25
Release Date
2018-05-16
Last Version Date
2024-05-08
Entry Detail
PDB ID:
6FL9
Keywords:
Title:
The active form of a pentameric ion channel (sTeLIC) gated by alkaline pH - Wild type 2.3 Angstrom resolution
Biological Source:
Source Organism(s):
endosymbiont of Tevnia jerichonana (Taxon ID: 1049564)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.30 Å
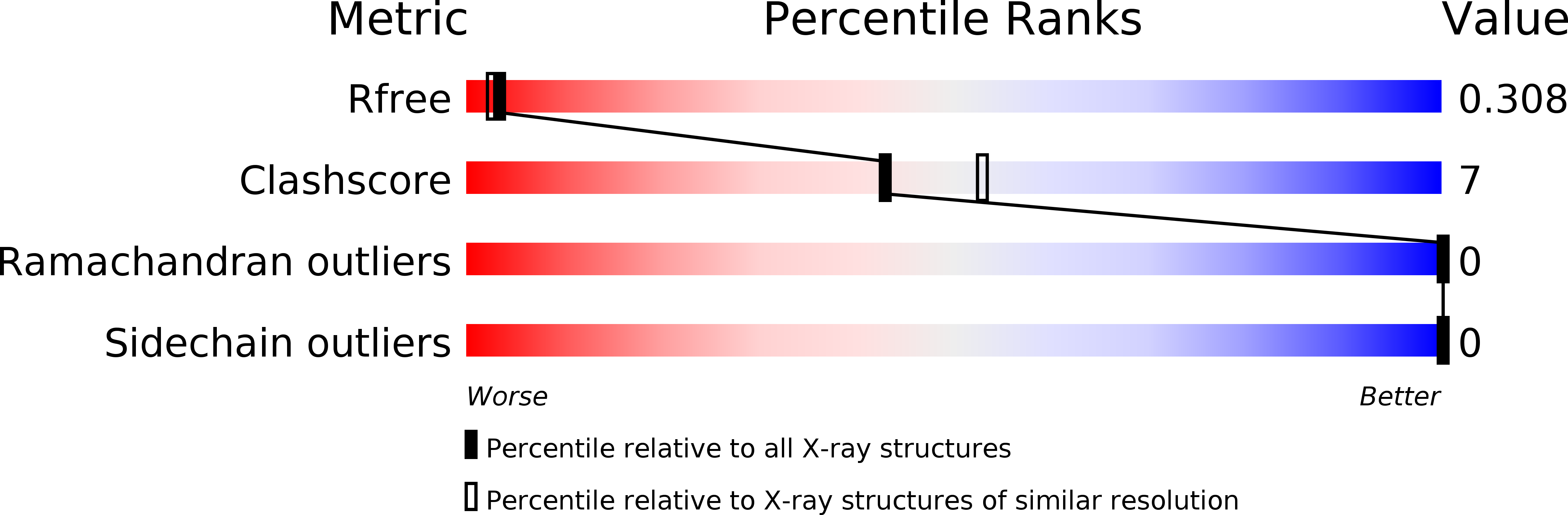
R-Value Free:
0.23
R-Value Work:
0.20
R-Value Observed:
0.21
Space Group:
C 1 2 1