Deposition Date
2017-10-25
Release Date
2018-06-13
Last Version Date
2024-10-09
Entry Detail
PDB ID:
6ET7
Keywords:
Title:
Activated heterodimer of the bacteriophytochrome regulated diguanylyl cyclase variant - S505V A526V - from Idiomarina species A28L
Biological Source:
Source Organism:
Idiomarina sp. A28L (Taxon ID: 1036674)
Host Organism:
Method Details:
Experimental Method:
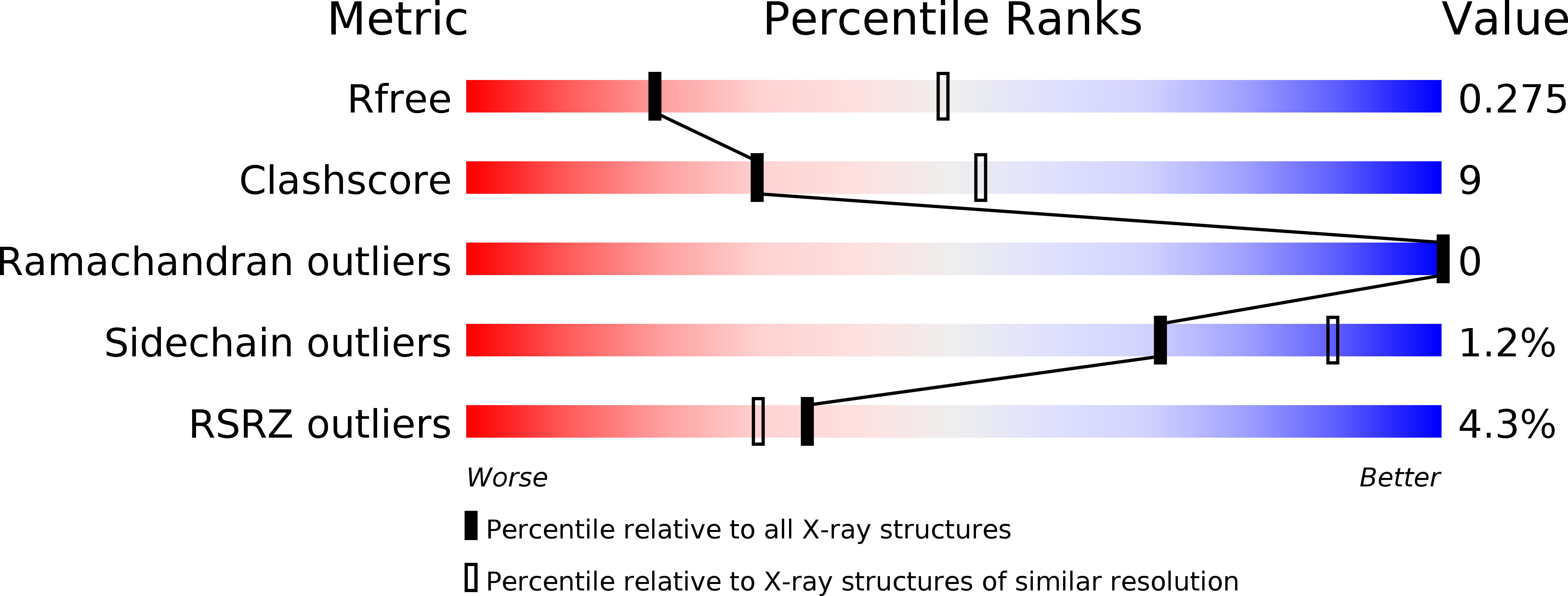
Resolution:
2.85 Å
R-Value Free:
0.27
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 21 21 21