Deposition Date
2018-08-20
Release Date
2019-04-03
Last Version Date
2024-10-09
Entry Detail
PDB ID:
6EGN
Keywords:
Title:
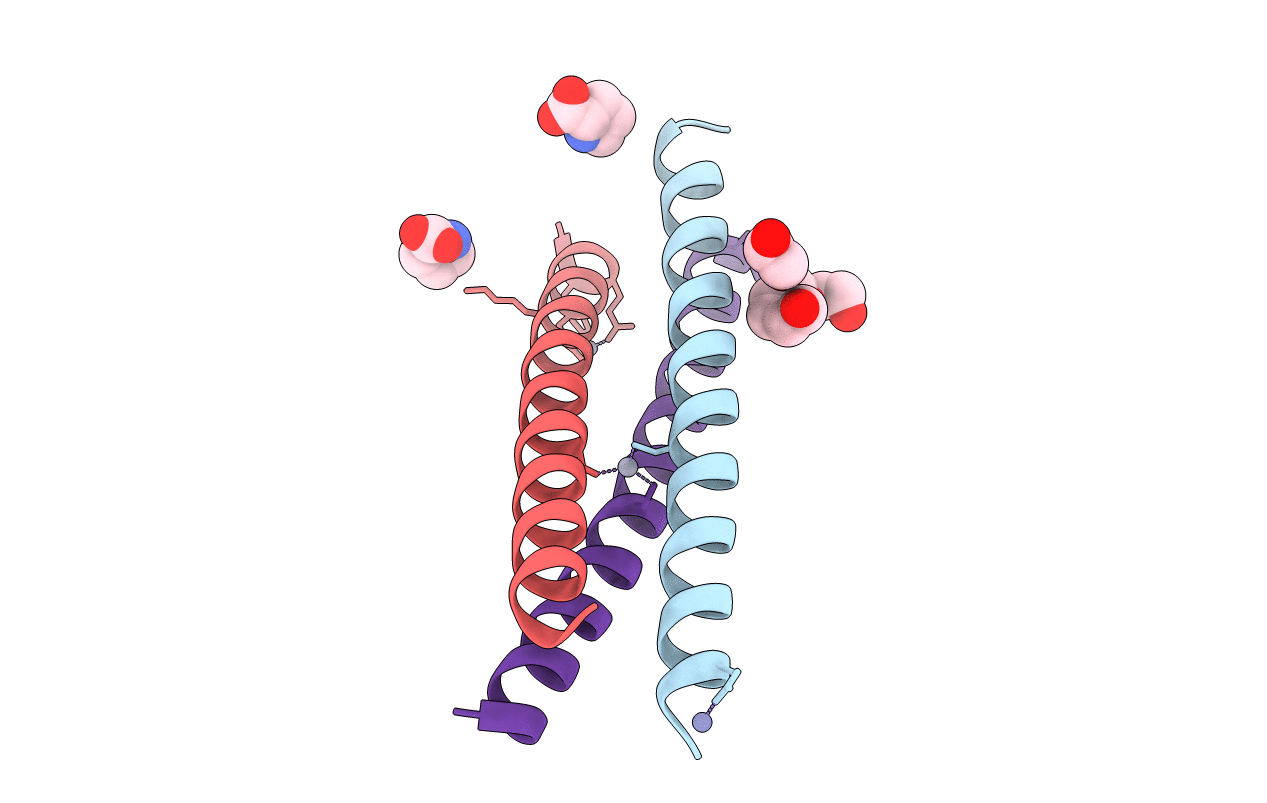
Crystal Structure of a Three-stranded Coiled Coil Peptide Containing a Trigonal Planar Hg(II)S3 Site Modified by D-Leu in the Second Coordination Sphere
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Method Details:
Experimental Method:
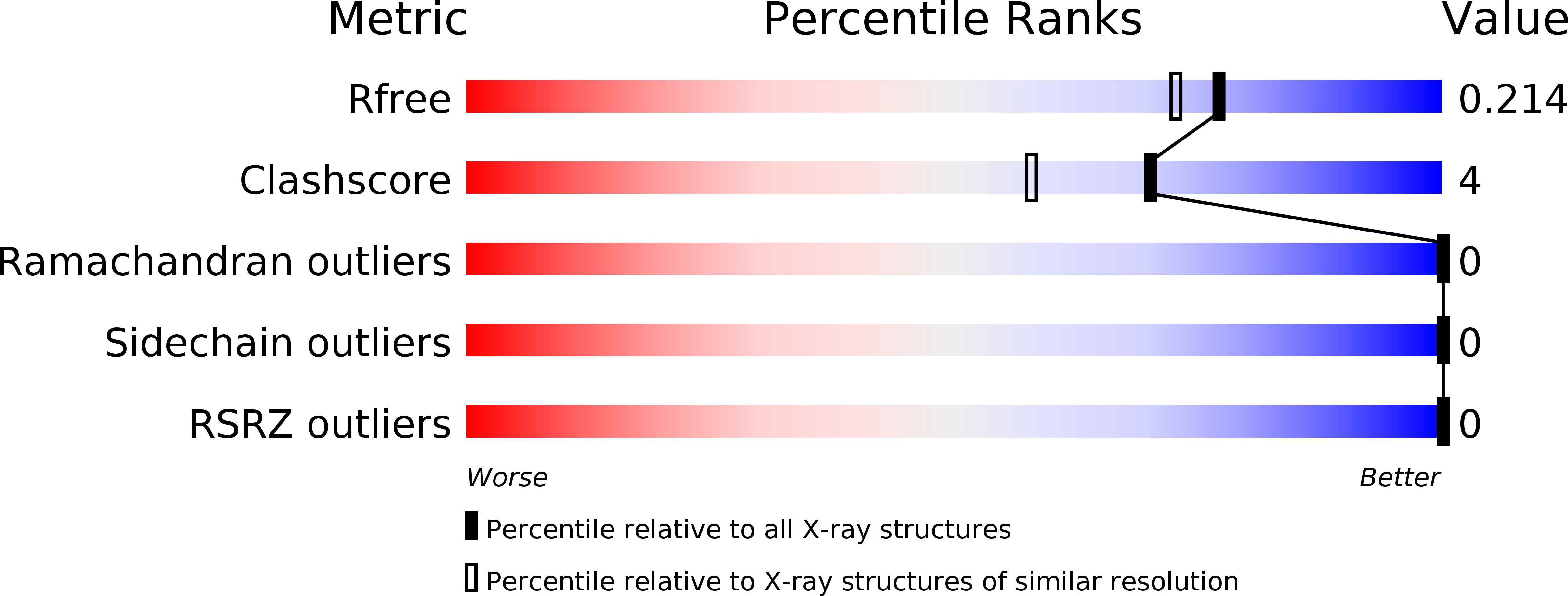
Resolution:
1.84 Å
R-Value Free:
0.20
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21