Deposition Date
2018-07-29
Release Date
2018-11-14
Last Version Date
2023-11-15
Entry Detail
PDB ID:
6E8I
Keywords:
Title:
Legionella Longbeachae LeSH (Llo2327) bound to phosphotyrosine
Biological Source:
Source Organism:
Host Organism:
Method Details:
Experimental Method:
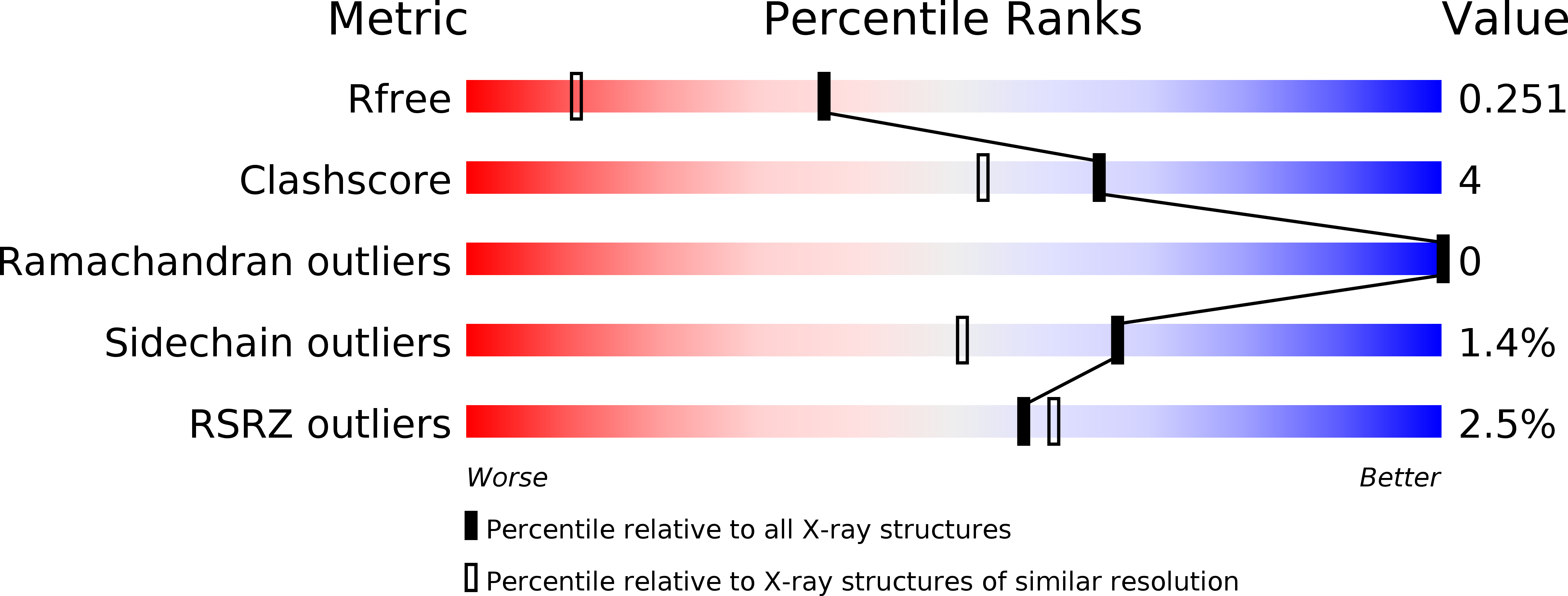
Resolution:
1.68 Å
R-Value Free:
0.25
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 21 21 21