Deposition Date
2018-07-26
Release Date
2018-11-07
Last Version Date
2023-10-11
Entry Detail
PDB ID:
6E7J
Keywords:
Title:
HIV-1 wild type protease with GRL-042-17A, 3-phenylhexahydro-2h-cyclopenta[d]oxazol-2-one with a bicyclic oxazolidinone scaffold as the P2 ligand
Biological Source:
Source Organism(s):
Human immunodeficiency virus 1 (Taxon ID: 11676)
Expression System(s):
Method Details:
Experimental Method:
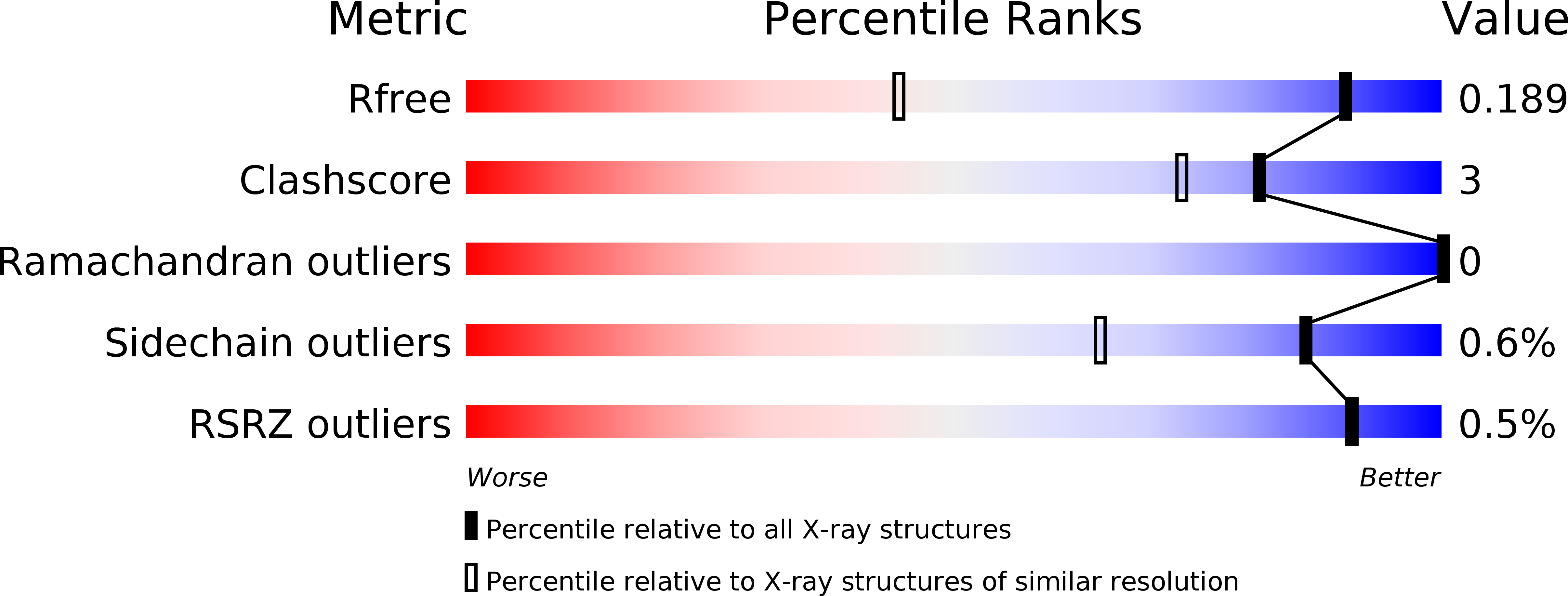
Resolution:
1.30 Å
R-Value Free:
0.18
R-Value Work:
0.14
R-Value Observed:
0.14
Space Group:
P 21 21 2