Deposition Date
2018-07-23
Release Date
2019-05-15
Last Version Date
2023-10-11
Entry Detail
PDB ID:
6E61
Keywords:
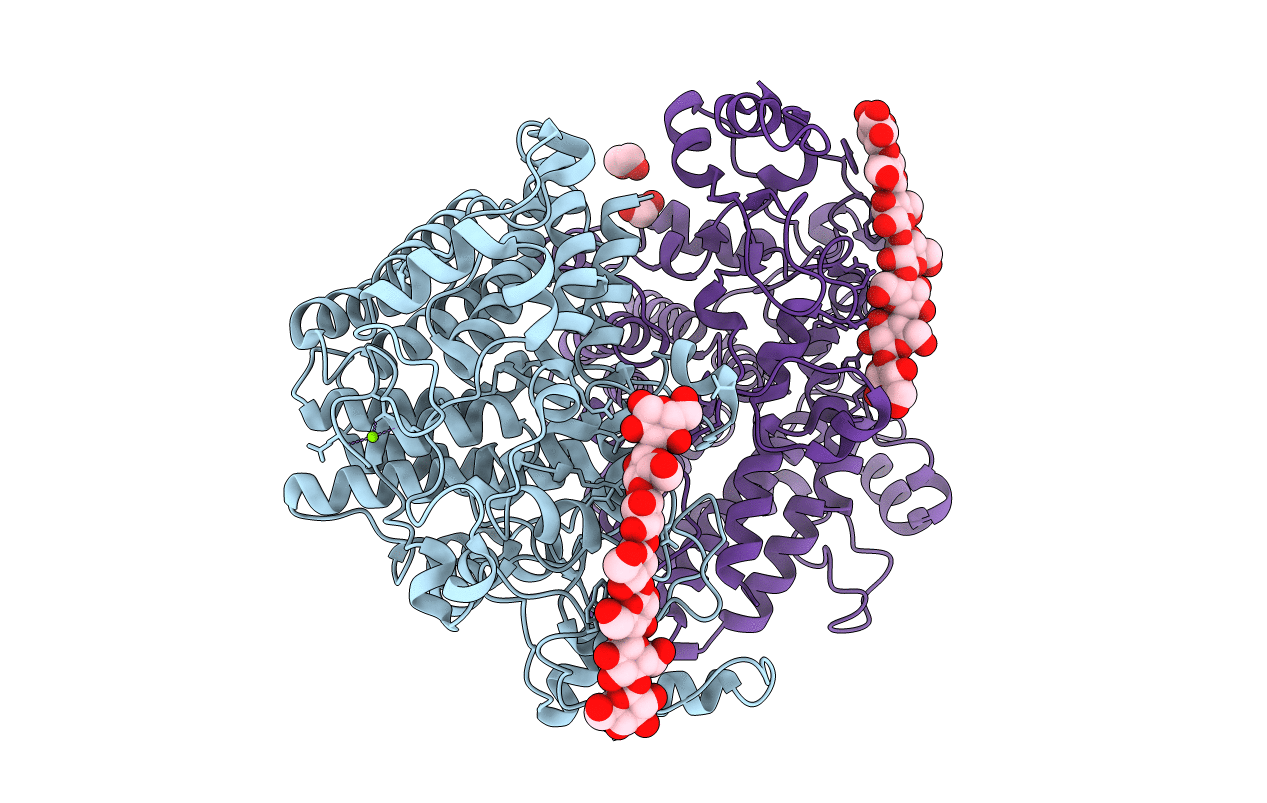
Title:
Bacteroides ovatus mixed-linkage glucan utilization locus (MLGUL) SGBP-A in complex with mixed-linkage heptasaccharide
Biological Source:
Source Organism(s):
Bacteroides ovatus (Taxon ID: 411476)
Expression System(s):
Method Details:
Experimental Method:
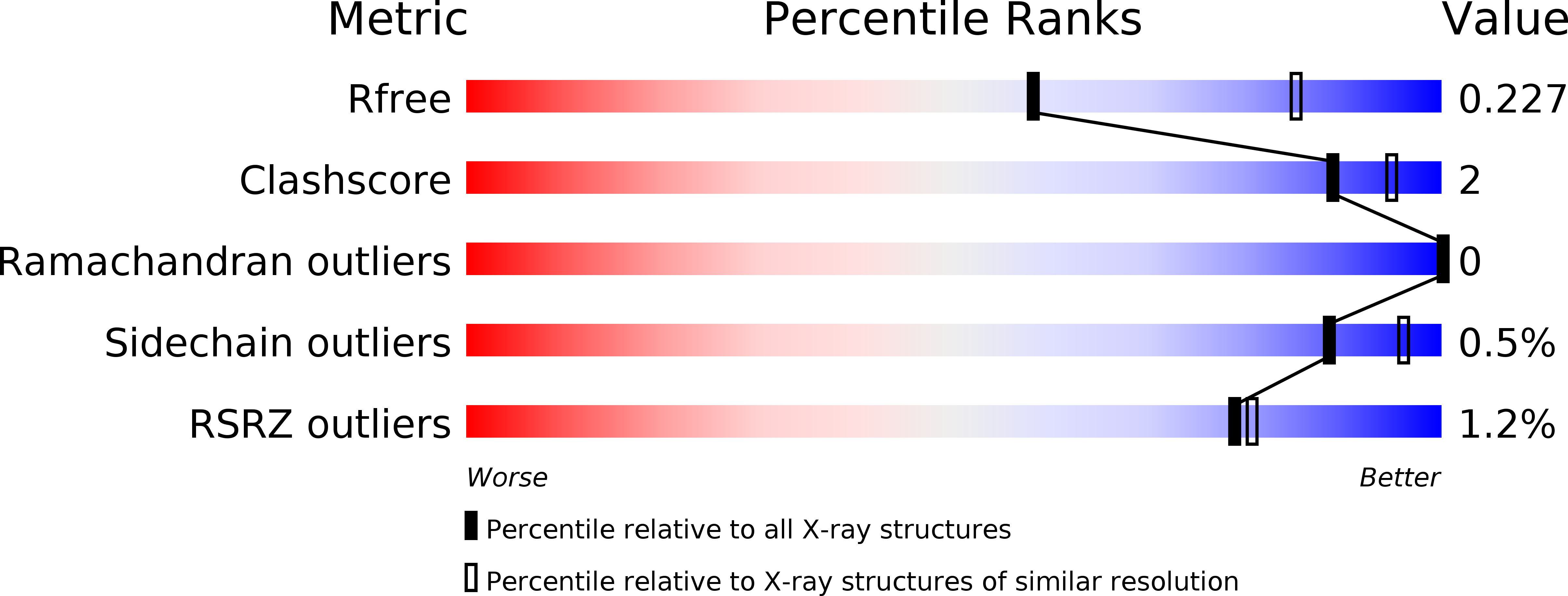
Resolution:
2.51 Å
R-Value Free:
0.22
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 21 21 21