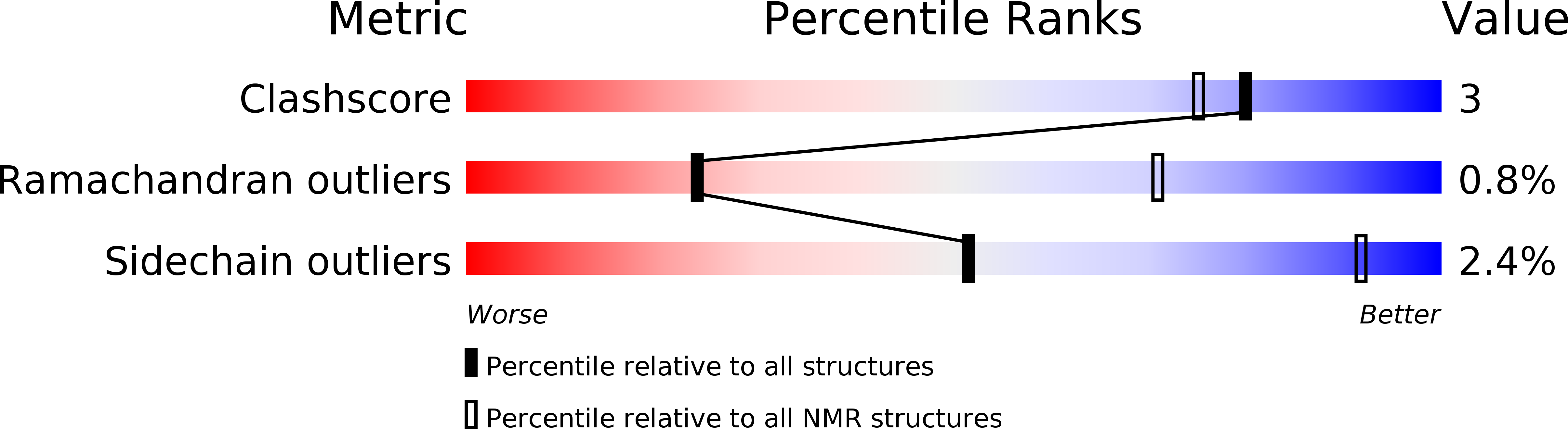
Deposition Date
2018-06-14
Release Date
2018-09-19
Last Version Date
2024-05-01
Entry Detail
Biological Source:
Source Organism(s):
Enterobacteria phage T7 (Taxon ID: 10760)
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
256
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy