Deposition Date
2018-05-30
Release Date
2019-04-10
Last Version Date
2024-11-20
Entry Detail
PDB ID:
6DKP
Keywords:
Title:
The complex among DMF5(alpha-D26Y, alpha-Y50A,beta-L98W) TCR, human Class I MHC HLA-A2 and MART-1(26-35)(A27L) peptide
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
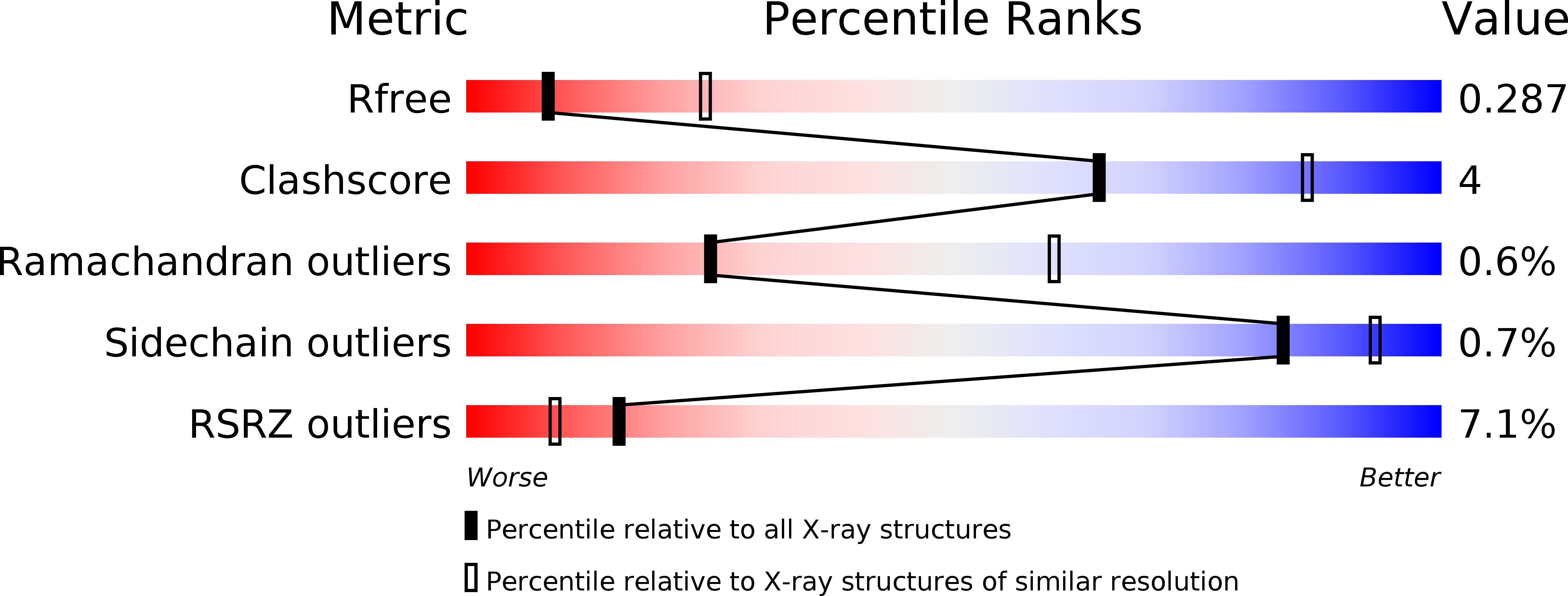
Resolution:
2.97 Å
R-Value Free:
0.28
R-Value Work:
0.22
R-Value Observed:
0.23
Space Group:
C 1 2 1