Deposition Date
2018-05-22
Release Date
2018-09-05
Last Version Date
2024-11-20
Entry Detail
PDB ID:
6DI5
Keywords:
Title:
CRYSTAL STRUCTURE OF BTK IN COMPLEX WITH COVALENT INHIBITOR
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
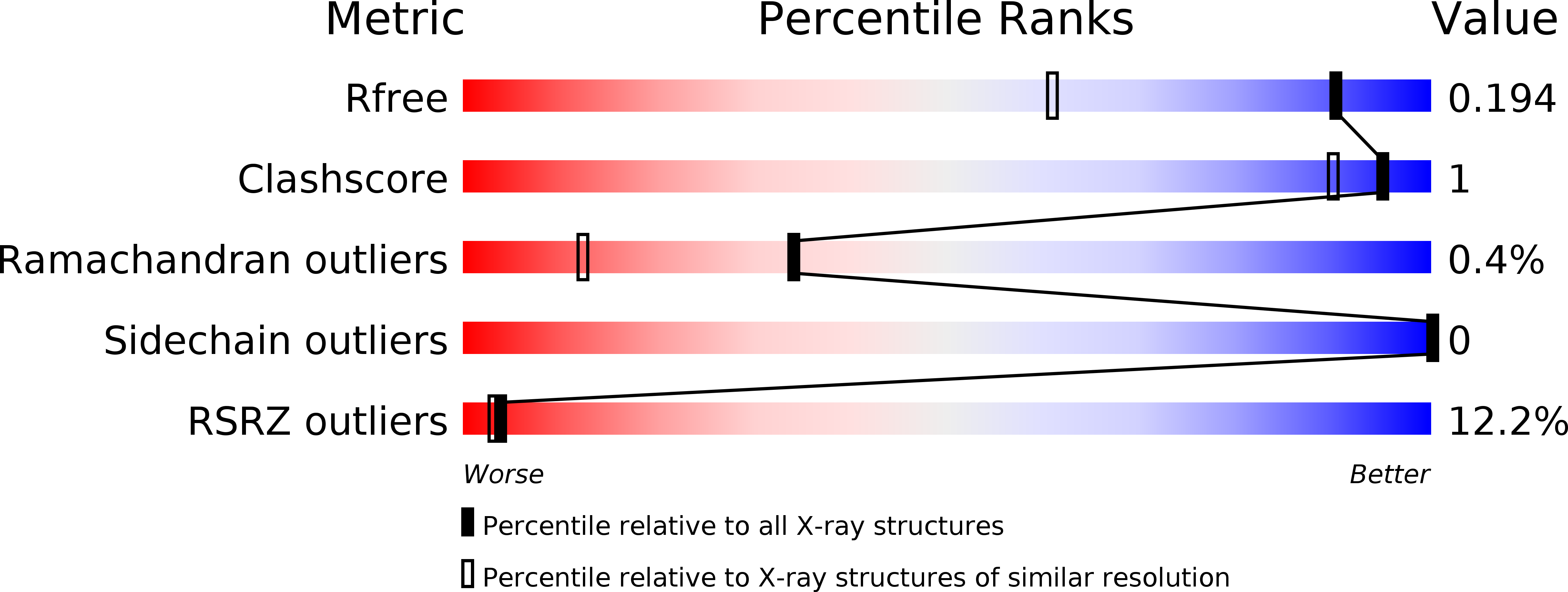
Resolution:
1.42 Å
R-Value Free:
0.19
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 21 21 2