Deposition Date
2018-04-04
Release Date
2019-04-17
Last Version Date
2023-10-04
Entry Detail
PDB ID:
6CXS
Keywords:
Title:
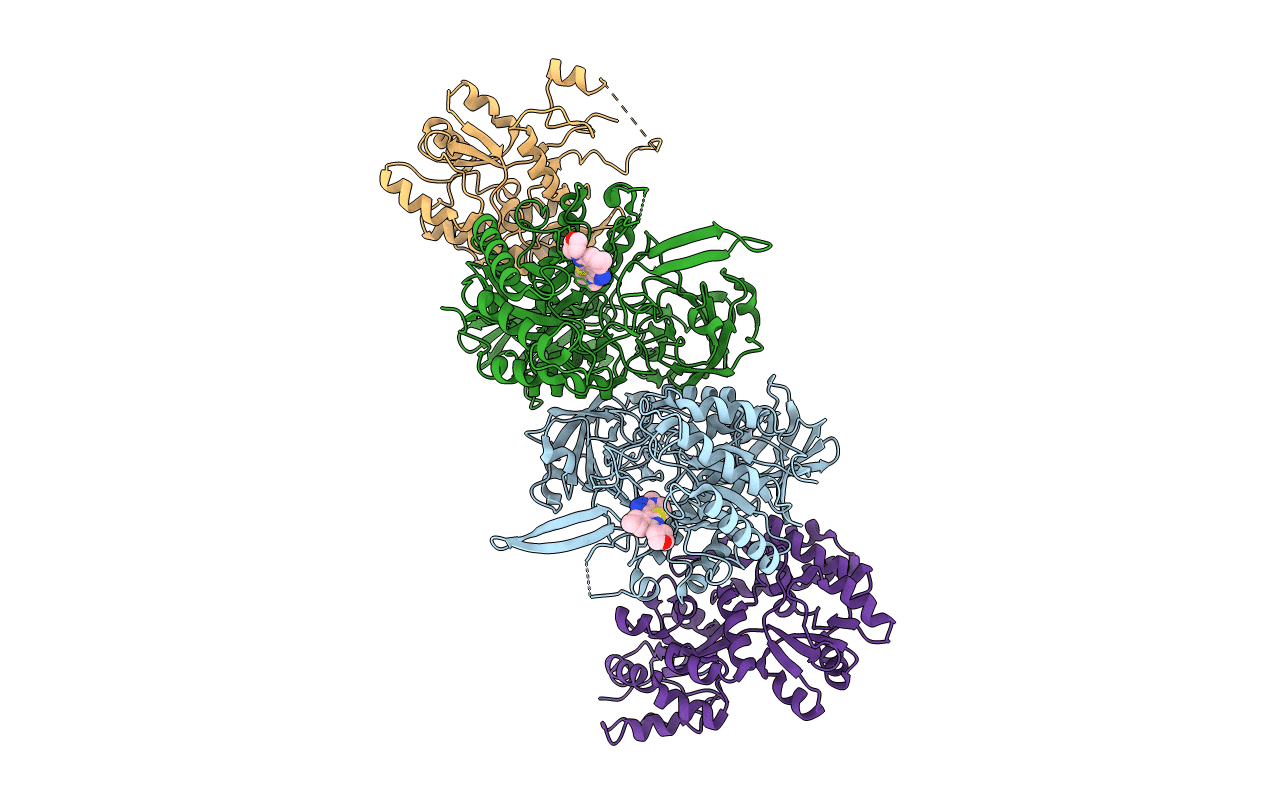
Crystal Structure of Clostridium perfringens beta-glucuronidase bound with a novel, potent inhibitor 4-(8-(piperazin-1-yl)-1,2,3,4-tetrahydro-[1,2,3]triazino[4',5':4,5]thieno[2,3-c]isoquinolin-5-yl)morpholine
Biological Source:
Source Organism(s):
Clostridium perfringens (strain 13 / Type A) (Taxon ID: 195102)
Escherichia coli (Taxon ID: 83333)
Escherichia coli (Taxon ID: 83333)
Expression System(s):
Method Details:
Experimental Method:
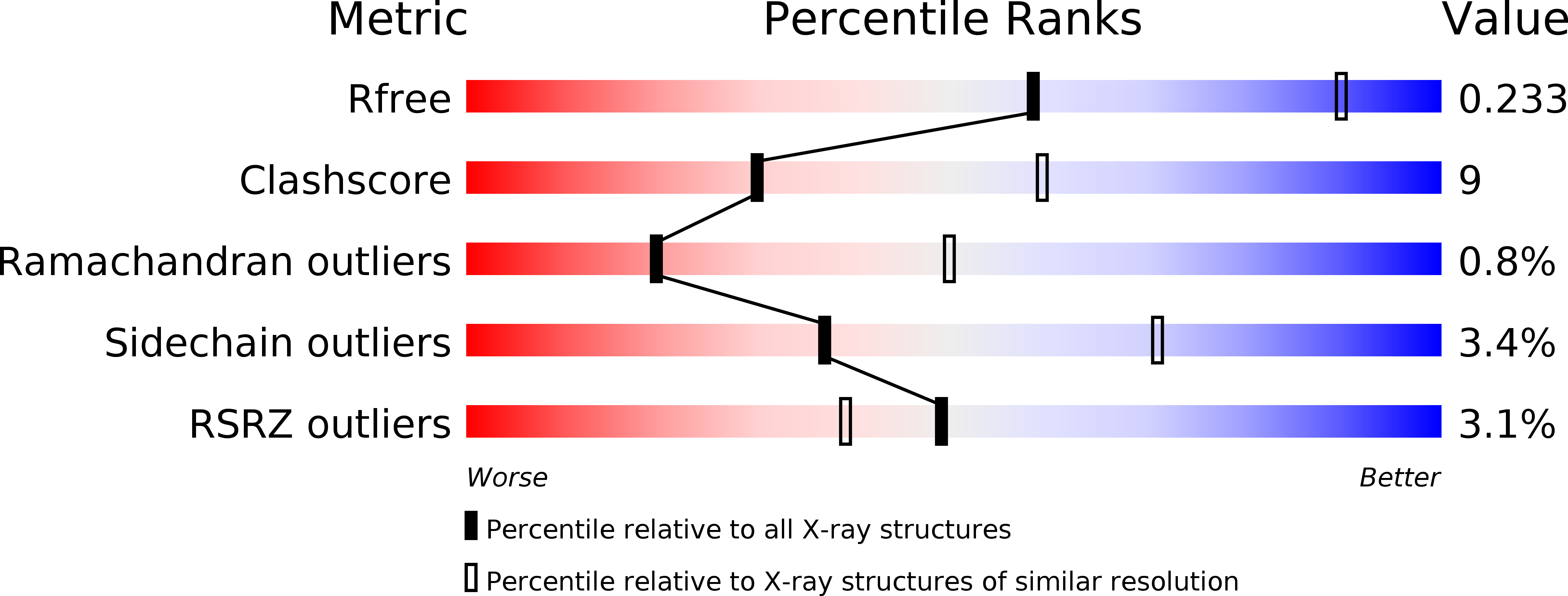
Resolution:
2.80 Å
R-Value Free:
0.23
R-Value Work:
0.19
R-Value Observed:
0.20
Space Group:
C 2 2 21