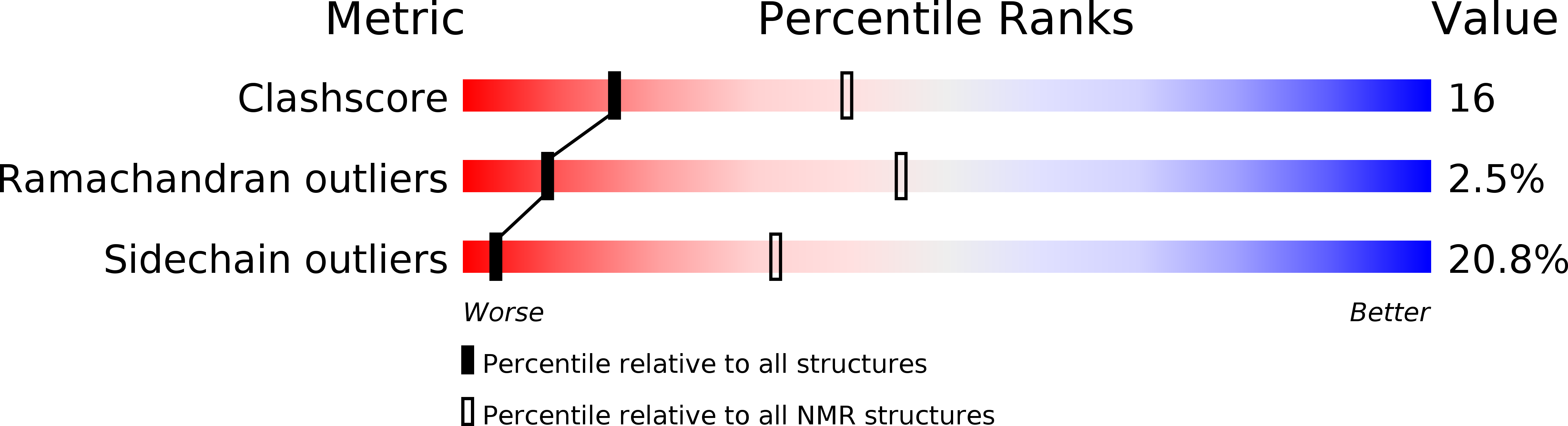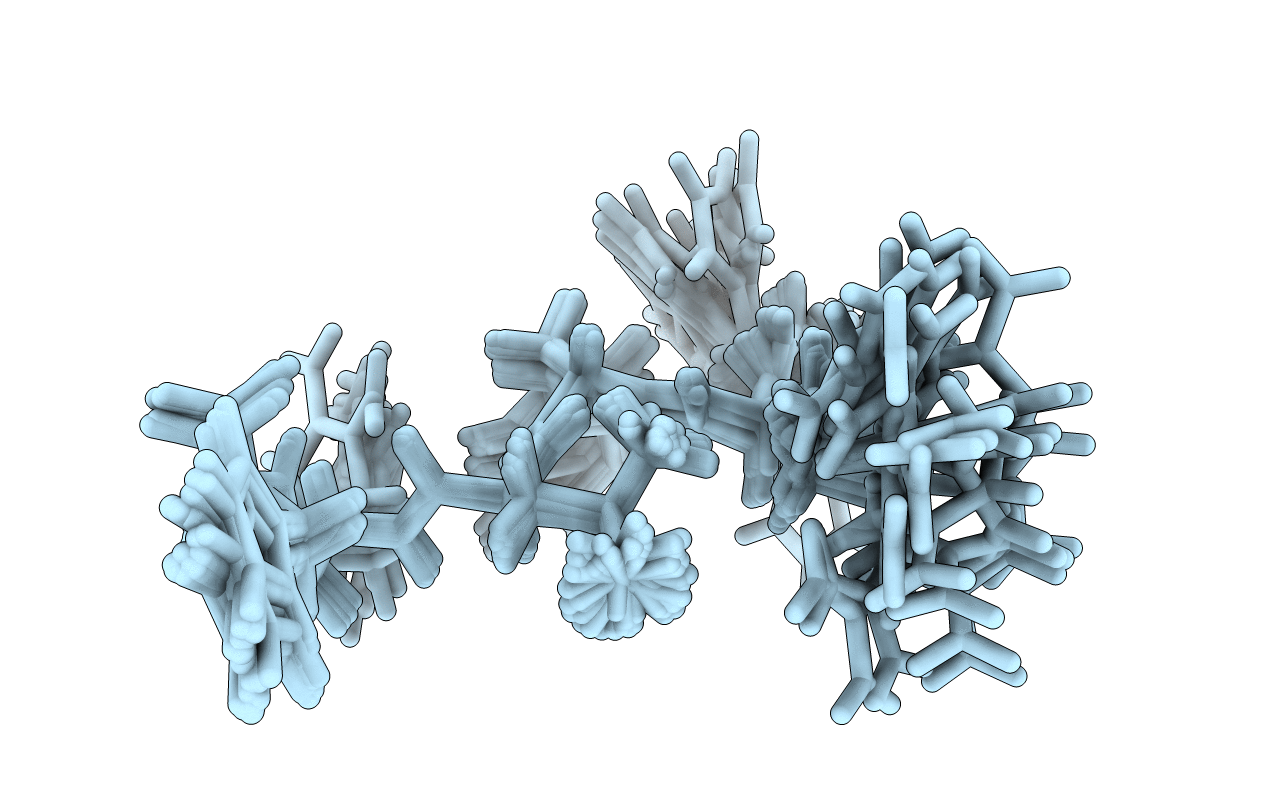
Deposition Date
2018-04-03
Release Date
2018-07-04
Last Version Date
2024-10-23
Entry Detail
PDB ID:
6CXR
Keywords:
Title:
HRFLRH peptide NMR structure in the presence of Zn(II)
Biological Source:
Source Organism(s):
Phyllomedusa centralis (Taxon ID: 536631)
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy