Deposition Date
2018-03-27
Release Date
2019-07-24
Last Version Date
2024-03-13
Entry Detail
PDB ID:
6CVB
Keywords:
Title:
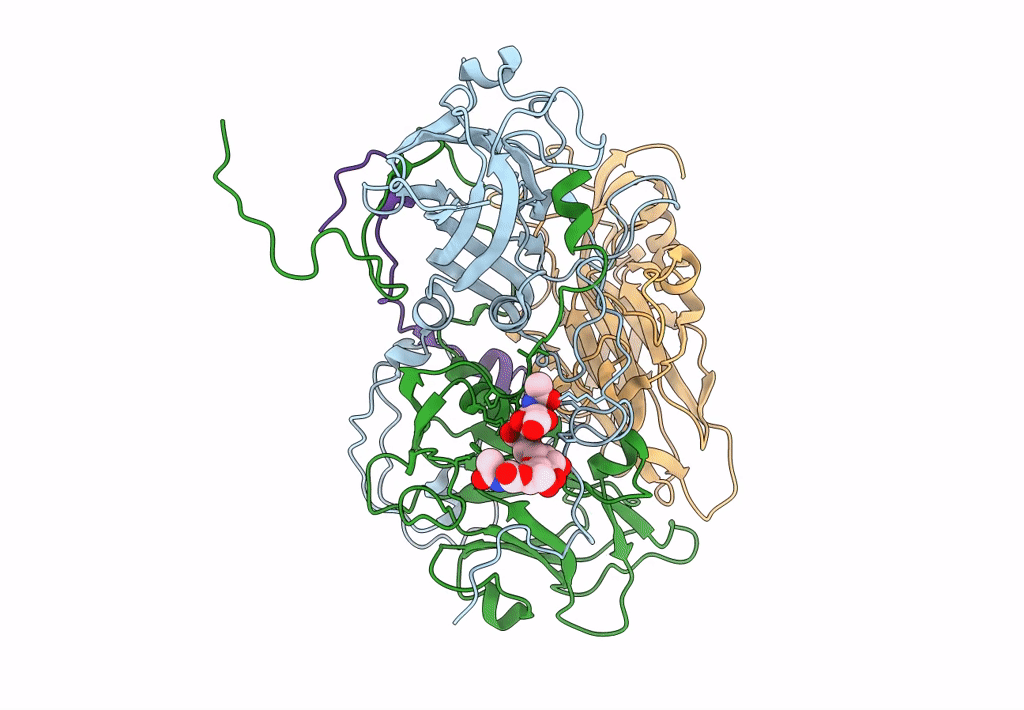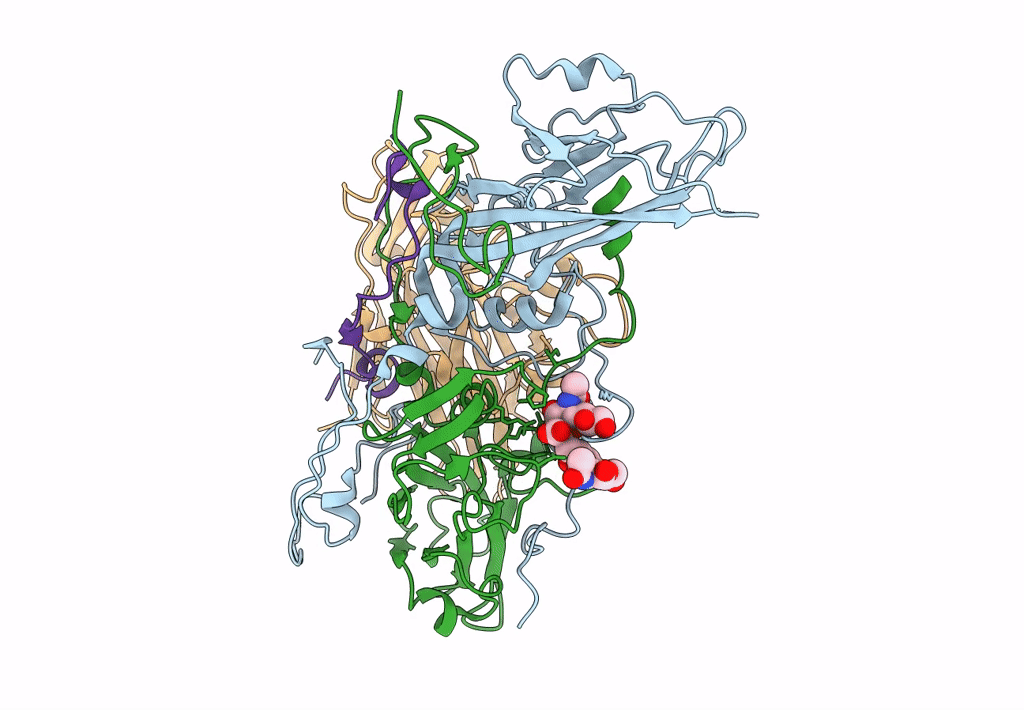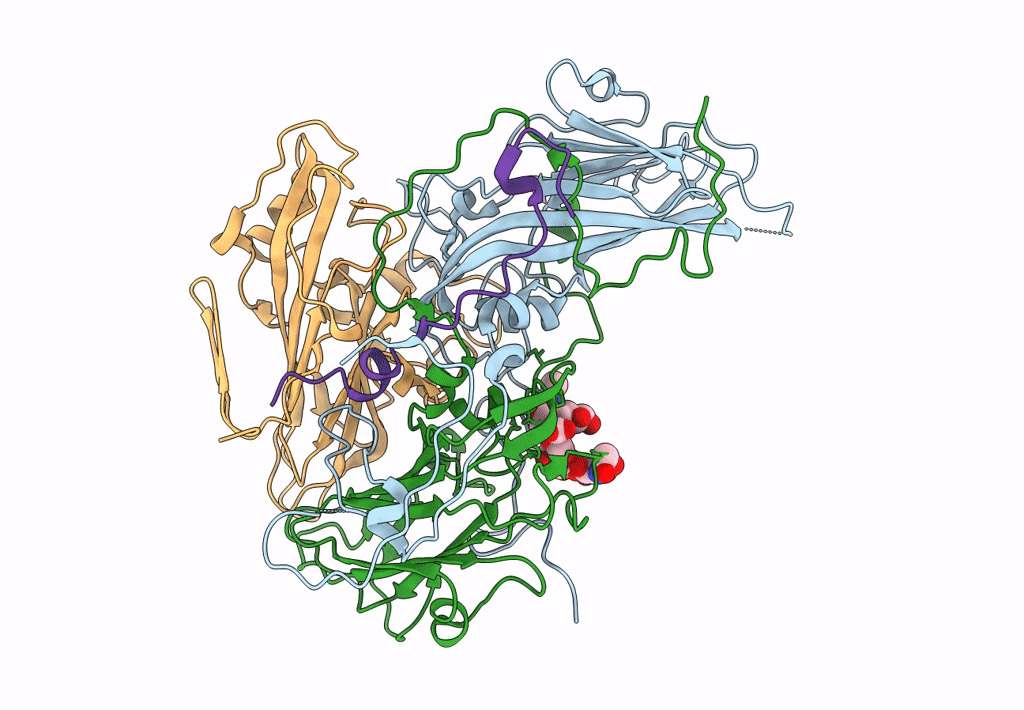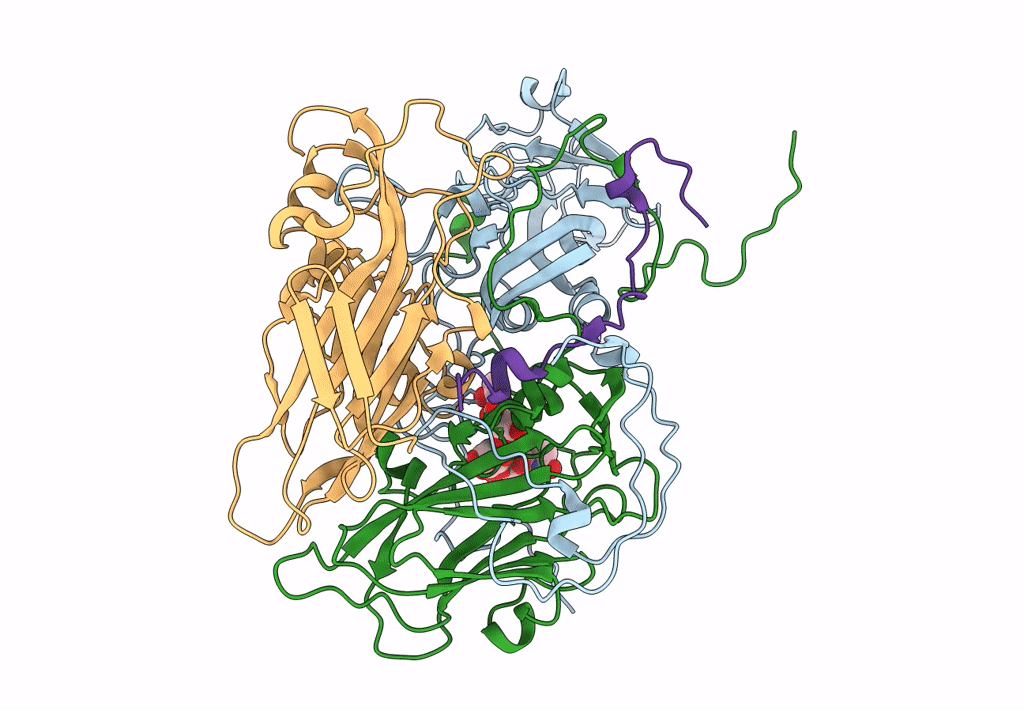
CryoEM structure of human enterovirus D68 in complex with 6'-sialyl-N-acetyllactosamine
Biological Source:
Source Organism(s):
Enterovirus D68 (Taxon ID: 42789)
Method Details:
Experimental Method:
Resolution:
2.43 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


