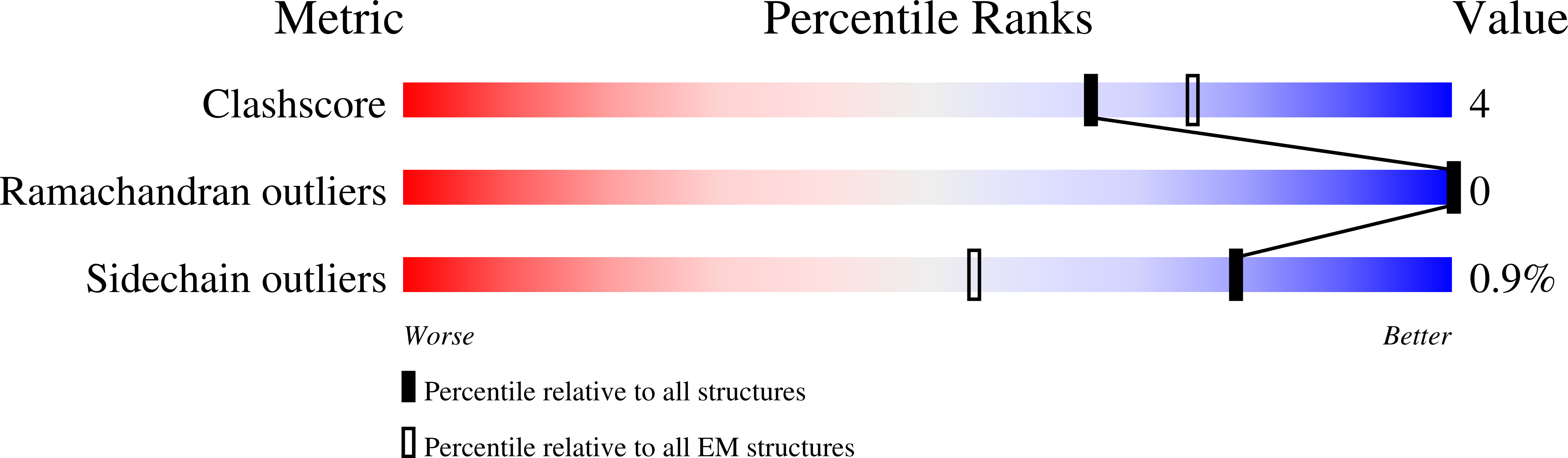
Deposition Date
2018-03-27
Release Date
2019-07-24
Last Version Date
2024-03-13
Entry Detail
PDB ID:
6CV1
Keywords:
Title:
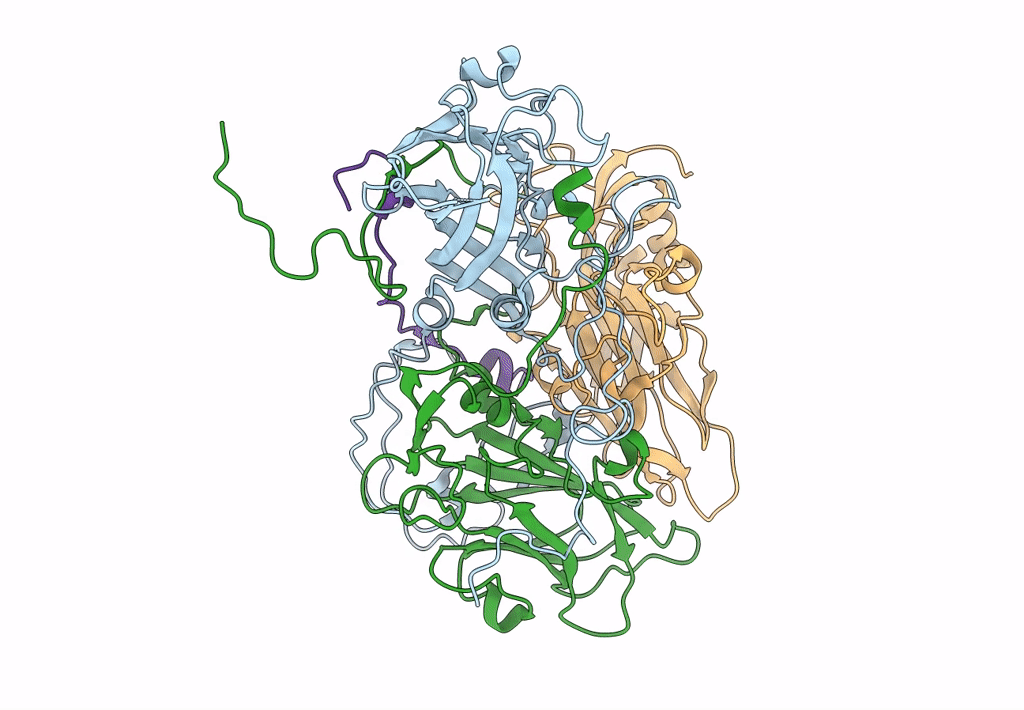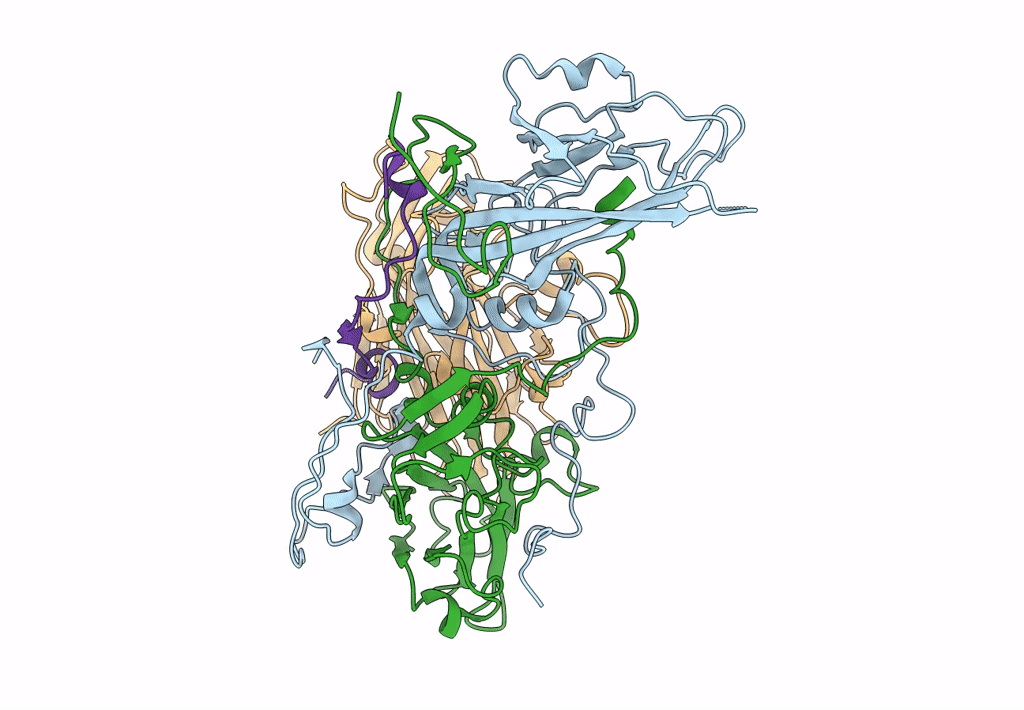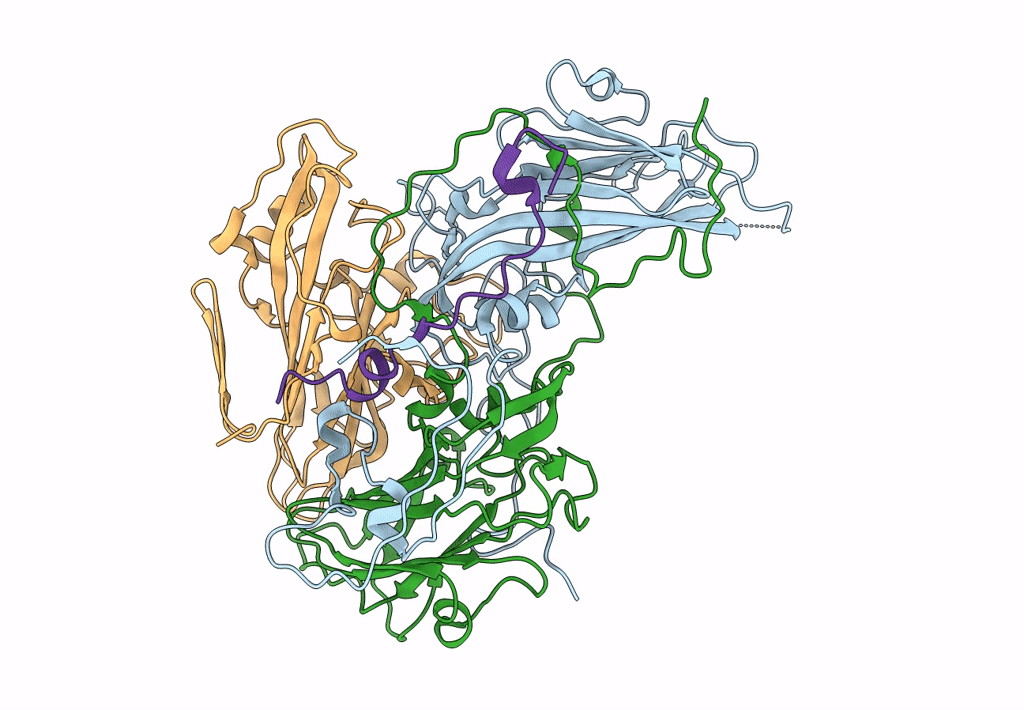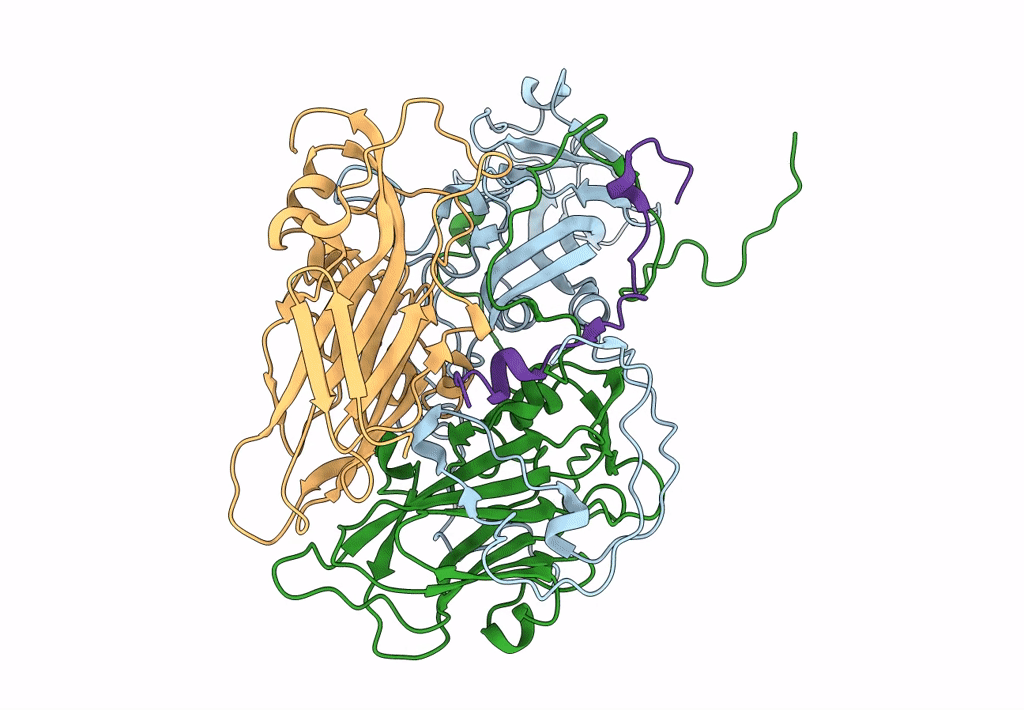
CryoEM structure of human enterovirus D68 full particle (after incubation with heparin-derived hexasaccharide)
Biological Source:
Source Organism(s):
Enterovirus D68 (Taxon ID: 42789)
Method Details:
Experimental Method:
Resolution:
2.76 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE