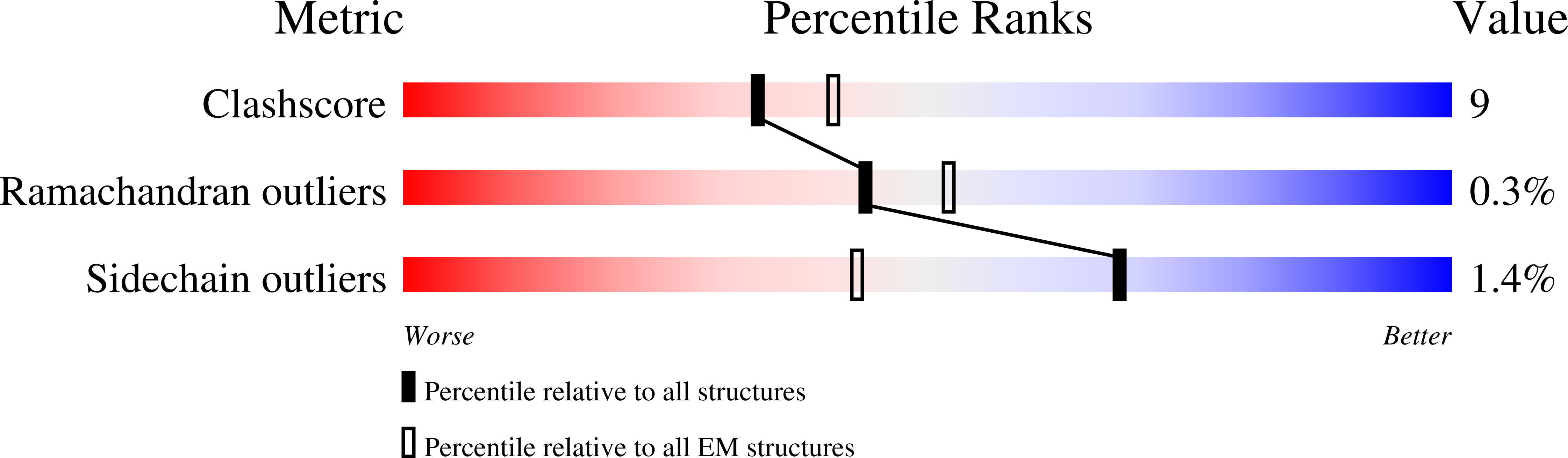Deposition Date
2018-03-13
Release Date
2018-04-11
Last Version Date
2024-10-09
Entry Detail
PDB ID:
6CP3
Keywords:
Title:
Monomer yeast ATP synthase (F1Fo) reconstituted in nanodisc with inhibitor of oligomycin bound.
Biological Source:
Source Organism(s):
Saccharomyces cerevisiae (Taxon ID: 559292)
Method Details:
Experimental Method:
Resolution:
3.80 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE