Deposition Date
2018-02-19
Release Date
2018-05-16
Last Version Date
2025-04-02
Entry Detail
PDB ID:
6CG3
Keywords:
Title:
Macrocyclic peptide derived from Abeta(17-36) - (ORN)LV(PHI)FAED(ORN)AII(2-nitrobenzylglycine)L(ORN)V
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Method Details:
Experimental Method:
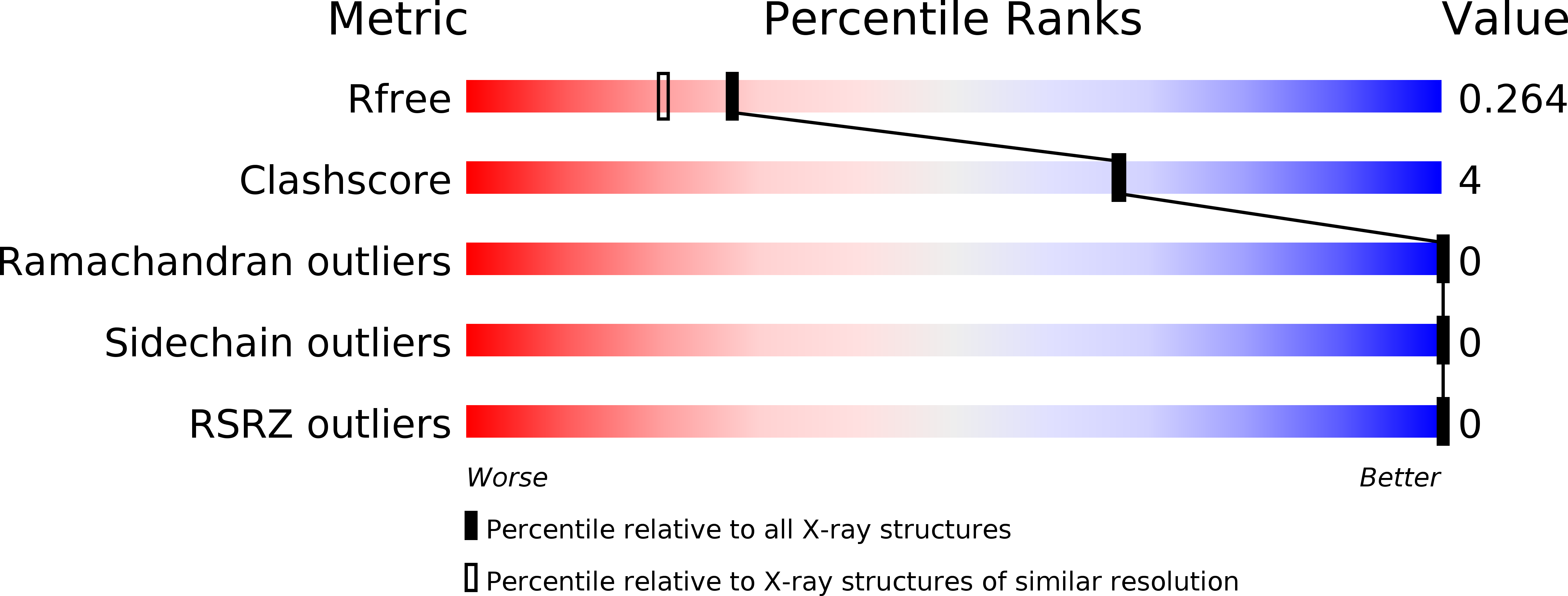
Resolution:
2.03 Å
R-Value Free:
0.25
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
P 42 3 2