Deposition Date
2018-01-01
Release Date
2018-06-27
Last Version Date
2024-11-13
Entry Detail
PDB ID:
6C0M
Keywords:
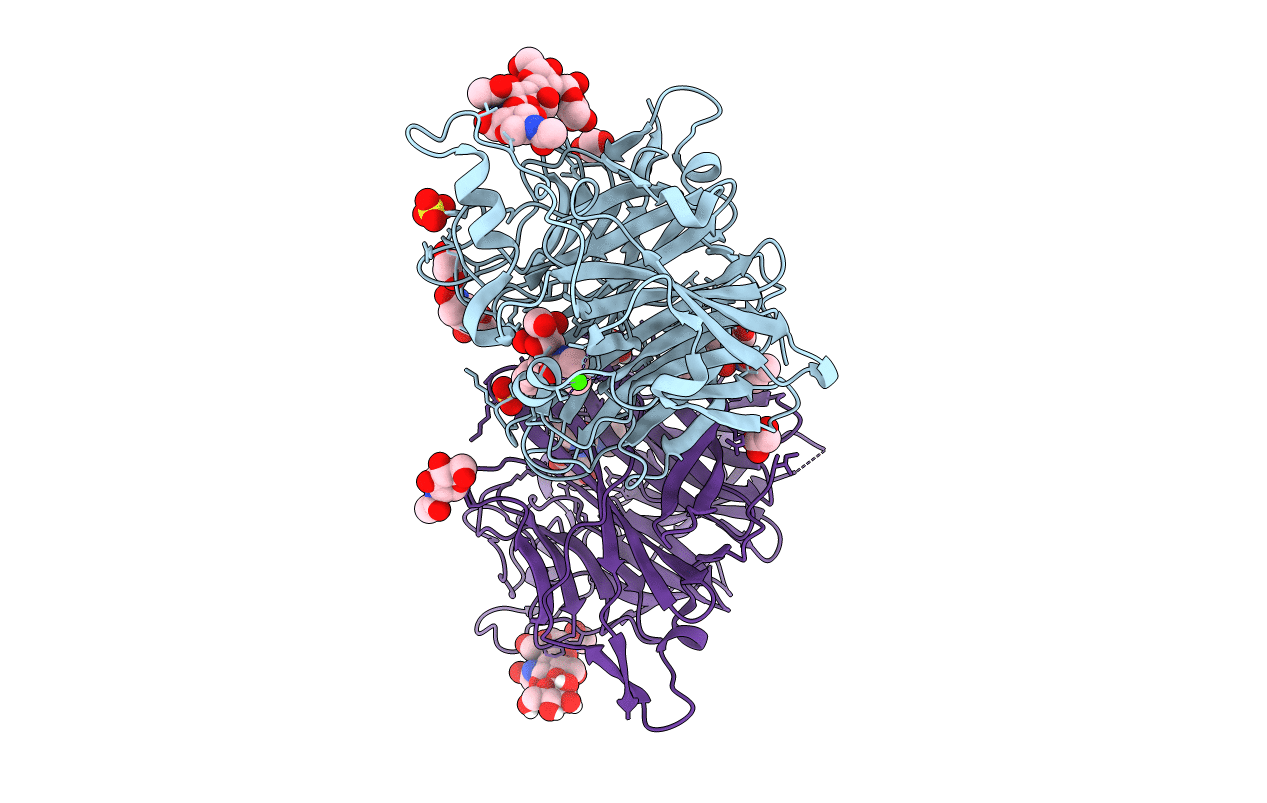
Title:
The synthesis, biological evaluation and structural insights of unsaturated 3-N-substituted sialic acids as probes of human parainfluenza virus-3 haemagglutinin-neuraminidase
Biological Source:
Source Organism(s):
Human respirovirus 3 (Taxon ID: 11216)
Expression System(s):
Method Details:
Experimental Method:
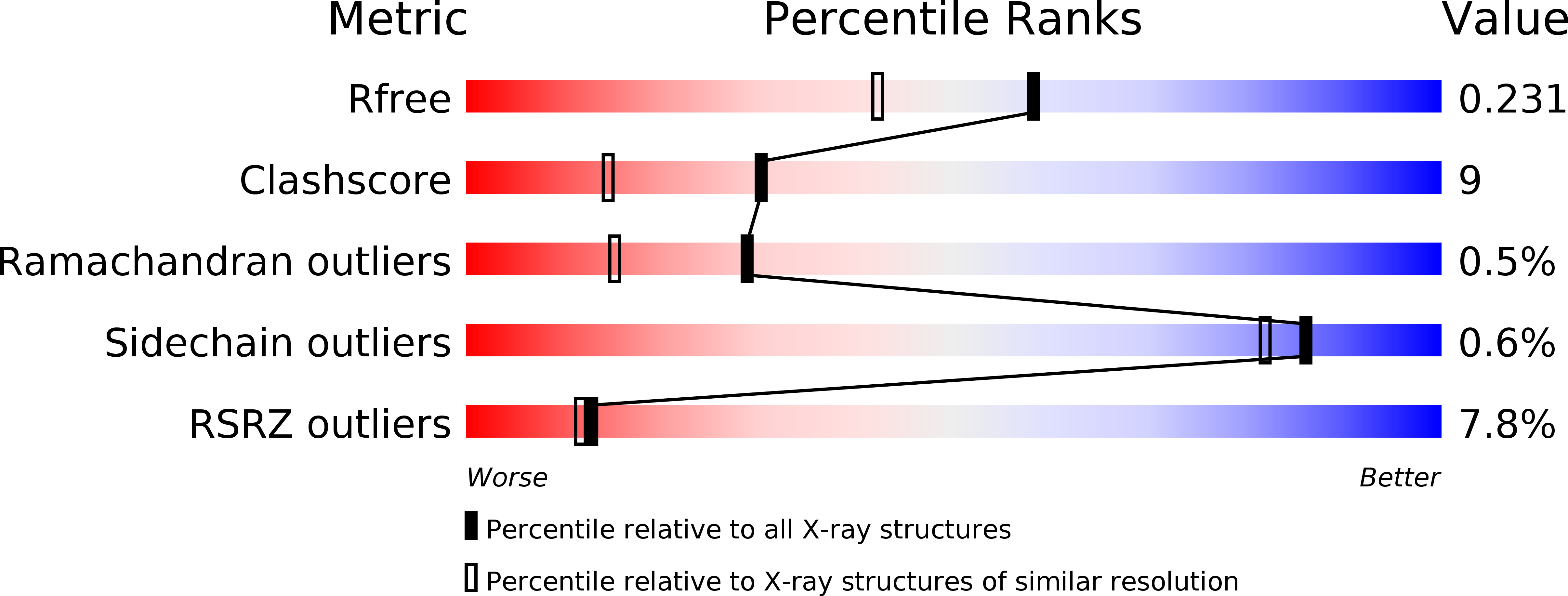
Resolution:
1.83 Å
R-Value Free:
0.22
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 21 21 21