Deposition Date
2017-10-31
Release Date
2018-04-18
Last Version Date
2024-11-06
Entry Detail
PDB ID:
6BHN
Keywords:
Title:
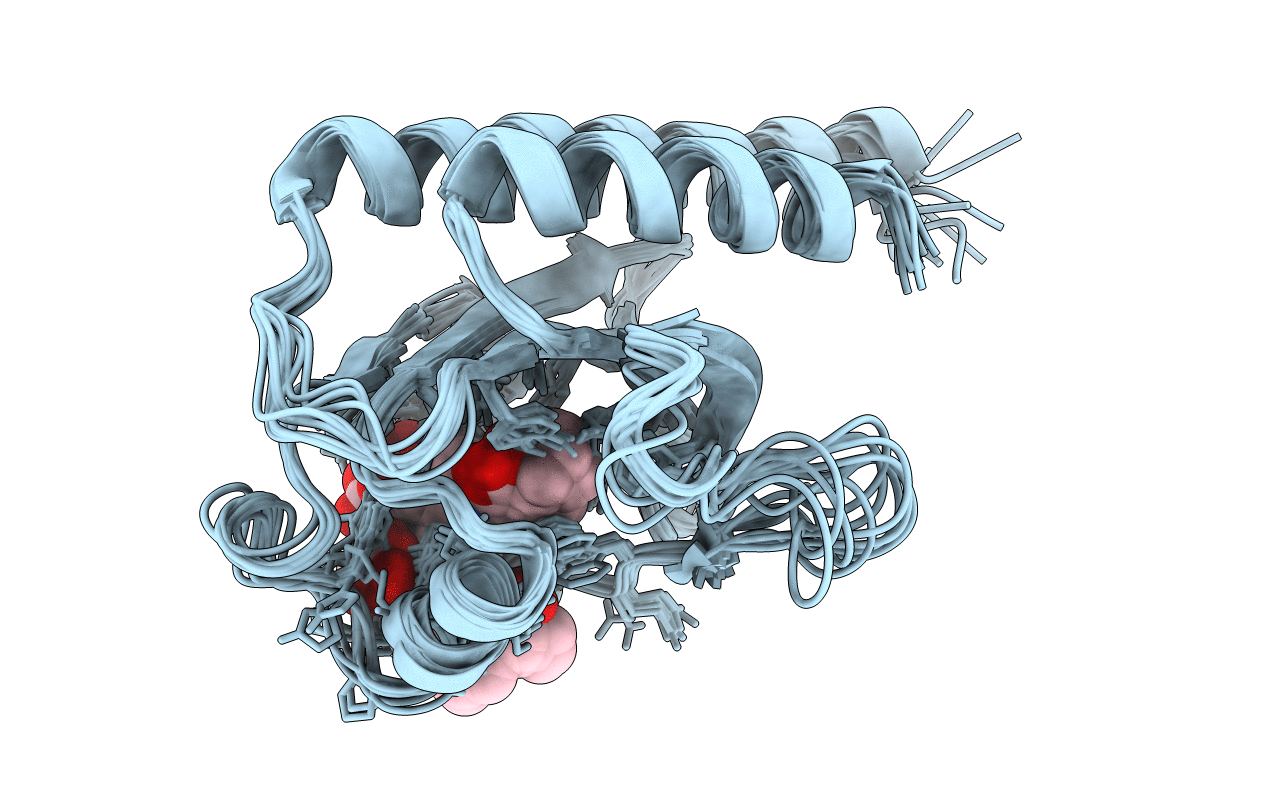
Red Light-Absorbing State of NpR6012g4, a Red/Green Cyanobacteriochrome
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
Conformers Calculated:
200
Conformers Submitted:
10
Selection Criteria:
structures with the lowest energy


