Deposition Date
2017-10-19
Release Date
2018-01-10
Last Version Date
2023-11-29
Entry Detail
PDB ID:
6BBQ
Keywords:
Title:
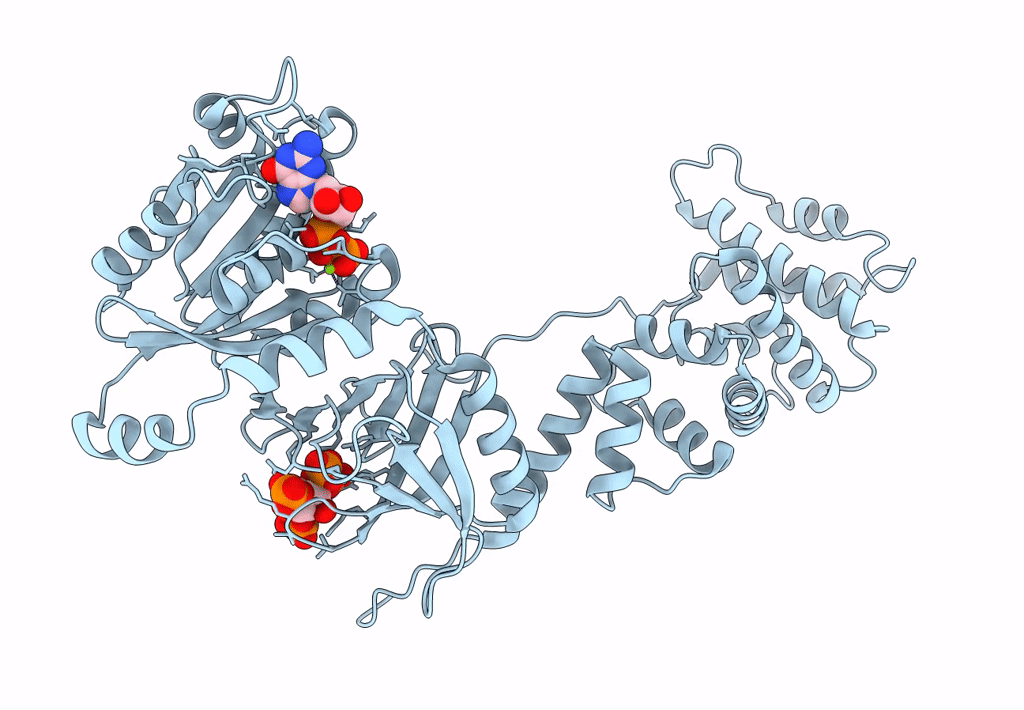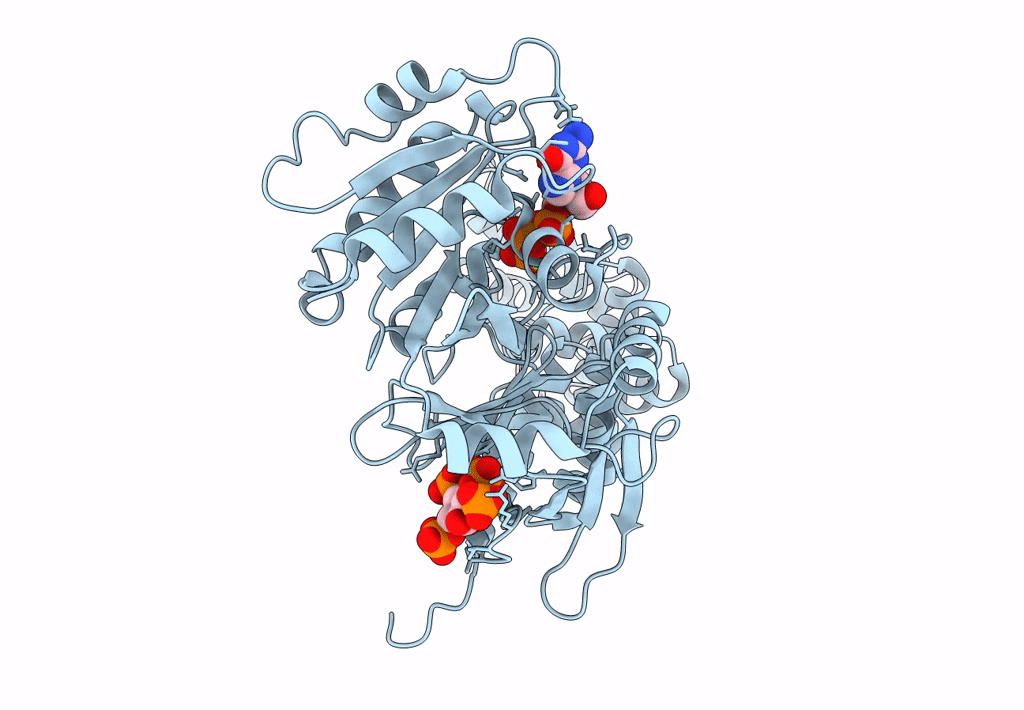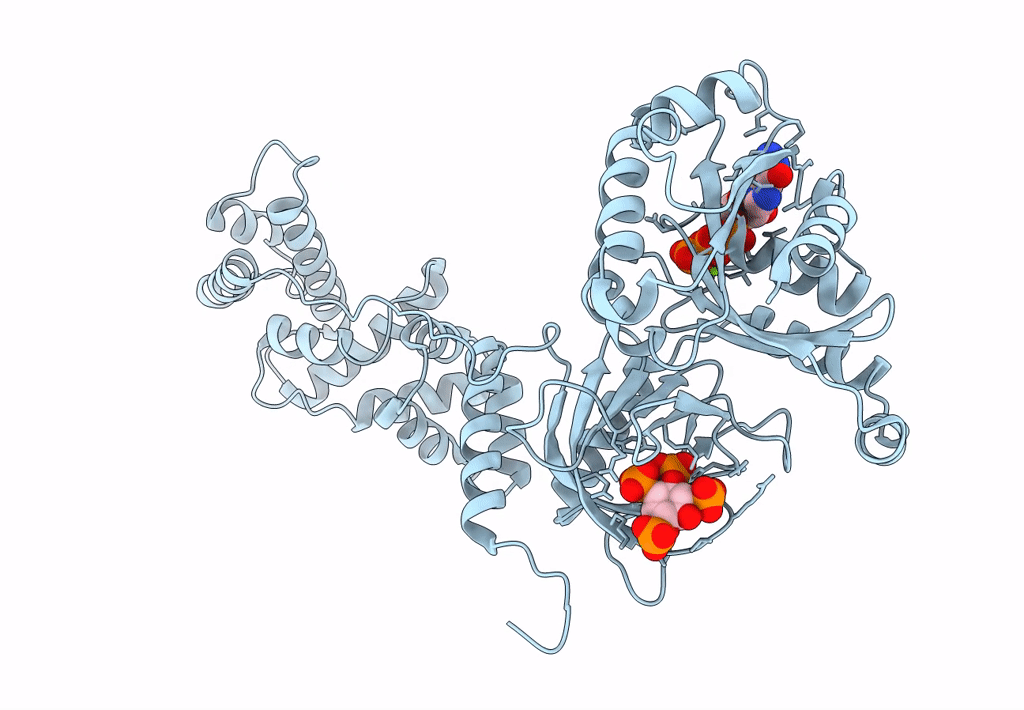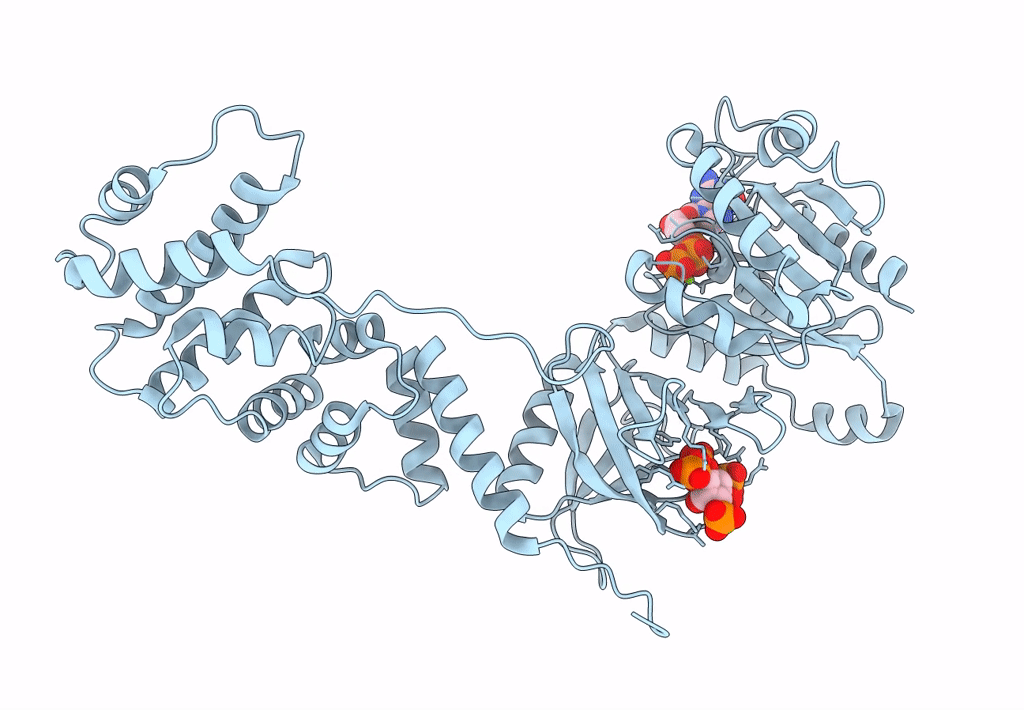
Model for extended volume of truncated monomeric Cytohesin-3 (Grp1; amino acids 63-399) E161A Arf6 Q67L fusion protein
Biological Source:
Source Organism(s):
Mus musculus (Taxon ID: 10090)
Homo sapiens (Taxon ID: 9606)
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
35.00 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE


