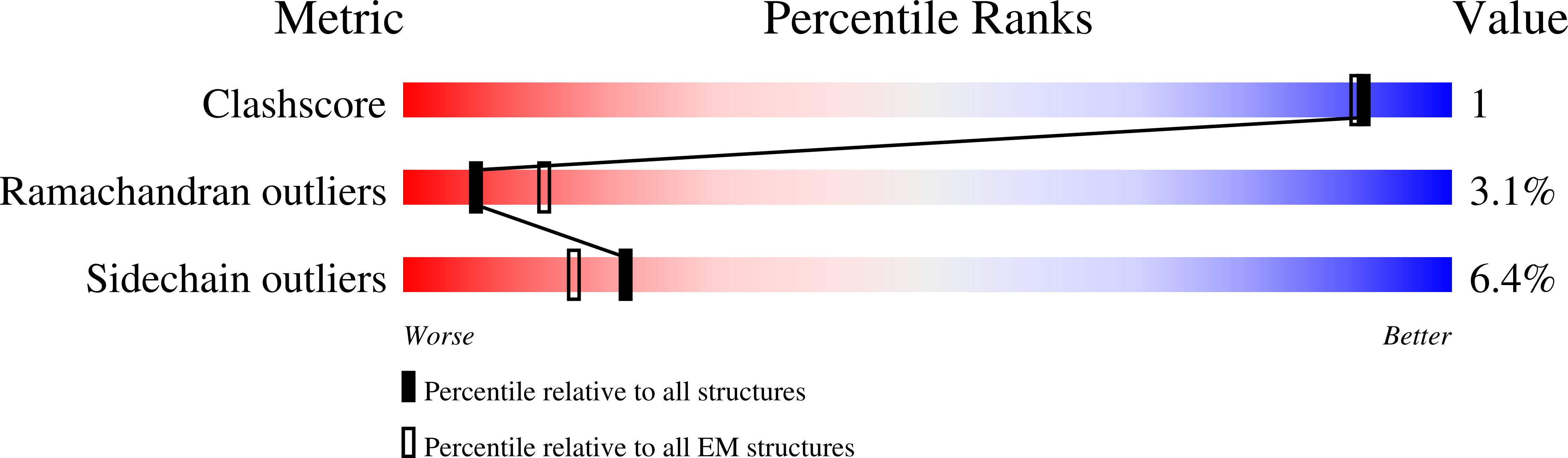
Deposition Date
2017-09-19
Release Date
2017-10-18
Last Version Date
2024-03-13
Entry Detail
PDB ID:
6B23
Keywords:
Title:
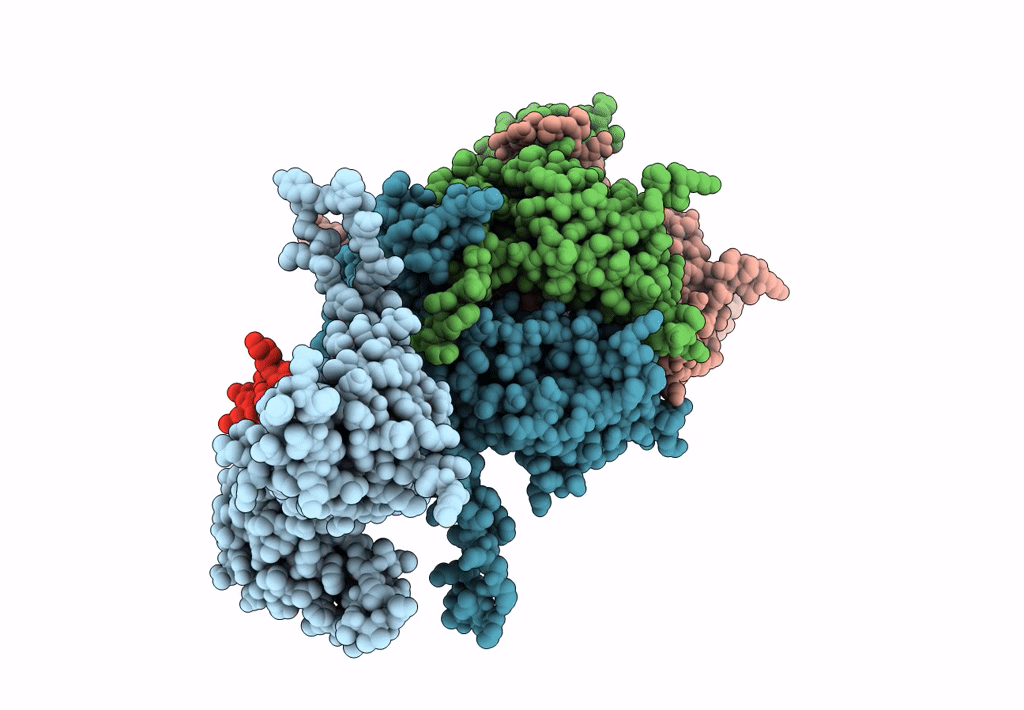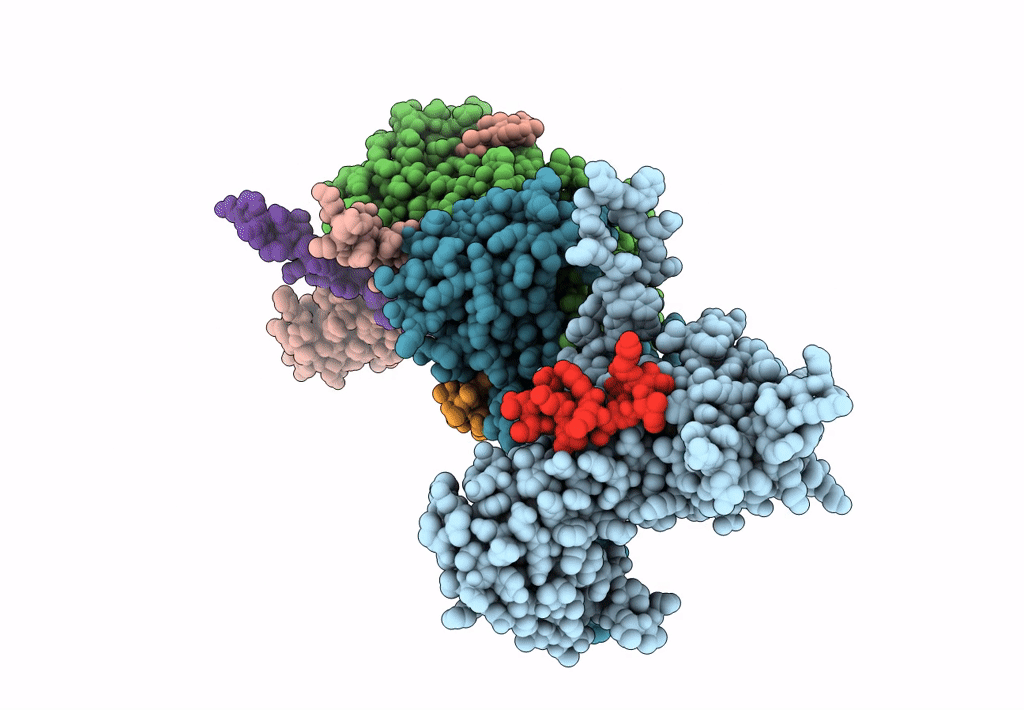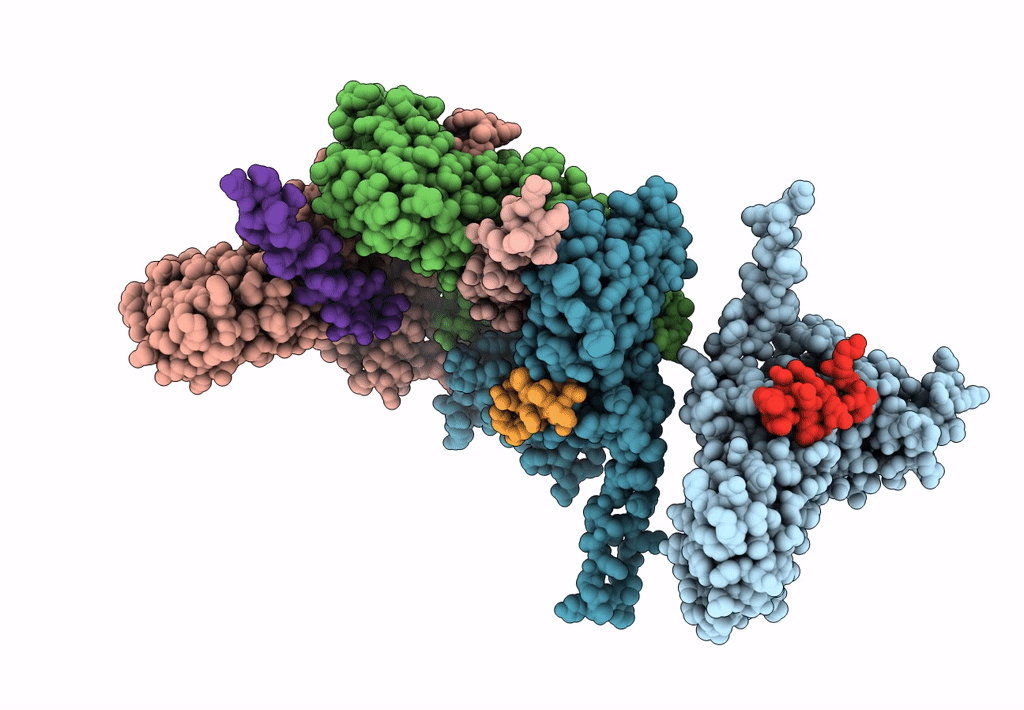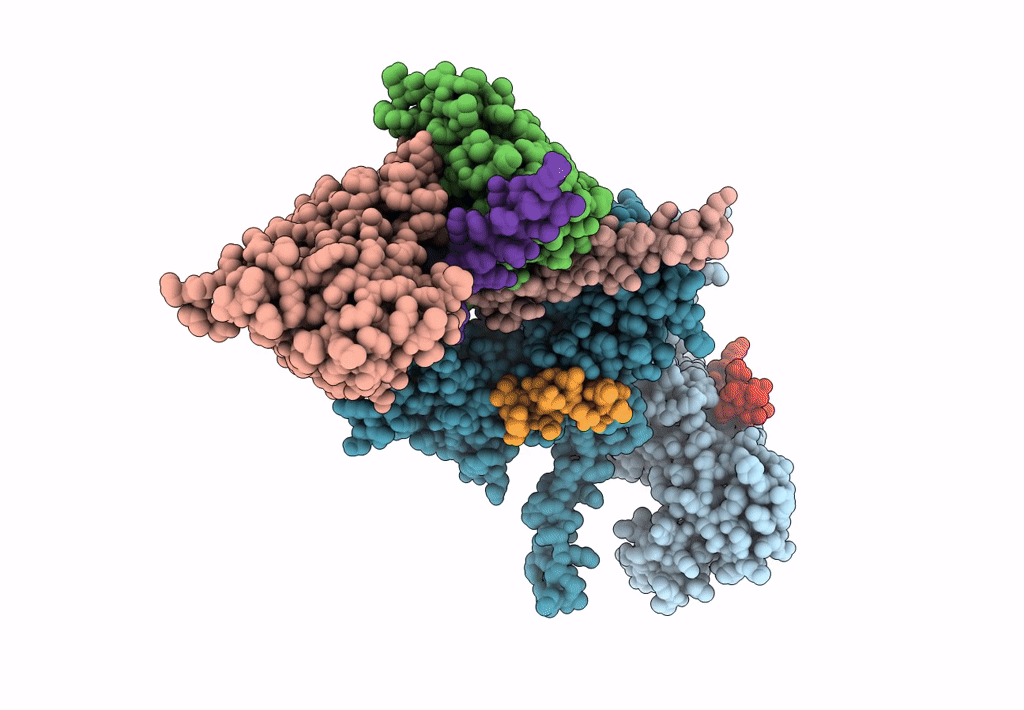
Capsid protein and C-terminal part of CpmB protein in the Staphylococcus aureus pathogenicity island 1 80alpha-derived procapsid
Biological Source:
Source Organism(s):
Staphylococcus phage 80alpha (Taxon ID: 53369)
Staphylococcus aureus (Taxon ID: 1280)
Staphylococcus aureus (Taxon ID: 1280)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
3.70 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE