Deposition Date
2017-08-29
Release Date
2018-08-08
Last Version Date
2024-11-06
Entry Detail
PDB ID:
6ATV
Keywords:
Title:
The molecular mechanisms by which NS1 of the 1918 Spanish influenza A virus hijack host protein-protein interactions
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Orthomyxoviridae (Taxon ID: 11308)
Orthomyxoviridae (Taxon ID: 11308)
Expression System(s):
Method Details:
Experimental Method:
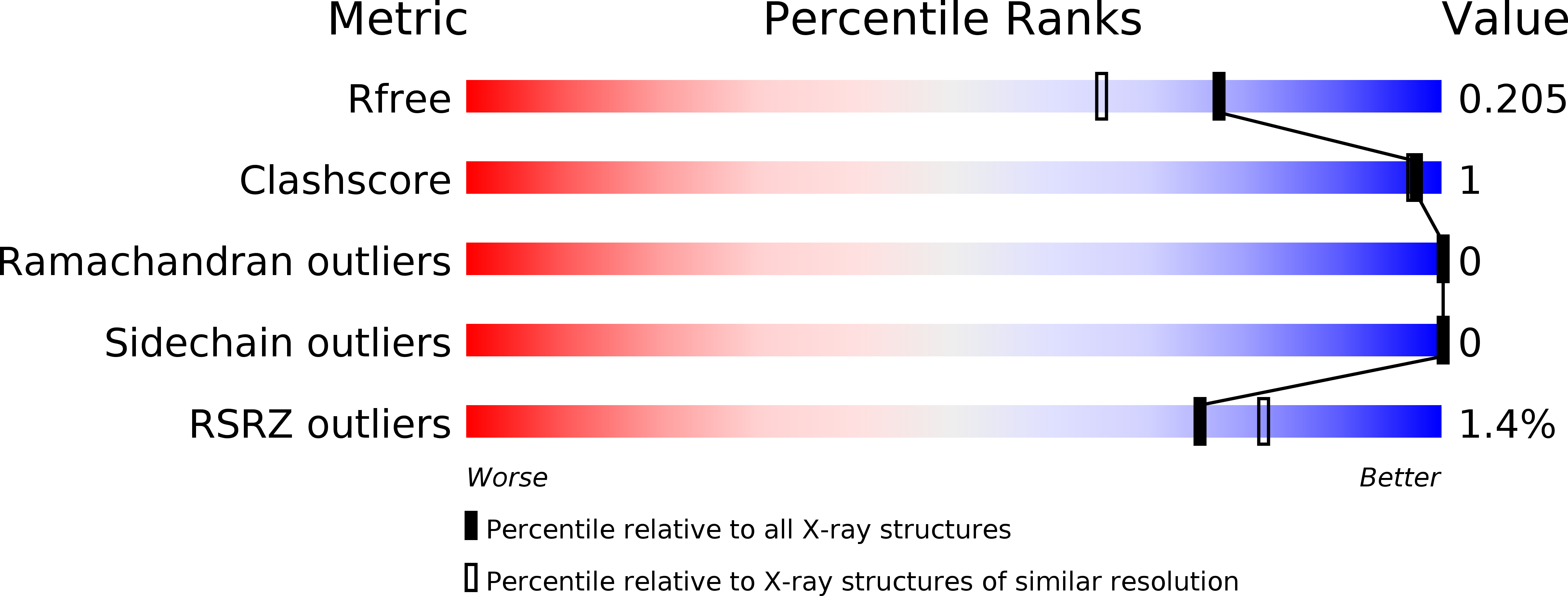
Resolution:
1.75 Å
R-Value Free:
0.20
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 61 2 2