Deposition Date
2017-08-23
Release Date
2018-02-28
Last Version Date
2024-10-23
Entry Detail
PDB ID:
6AS8
Keywords:
Title:
F9 pilus adhesin FmlH lectin domain from E. coli UTI89 co-crystallized with ortho-biphenyl-2'-carboxyl N-acetyl-beta-galactosaminoside
Biological Source:
Source Organism(s):
Escherichia coli (Taxon ID: 364106)
Expression System(s):
Method Details:
Experimental Method:
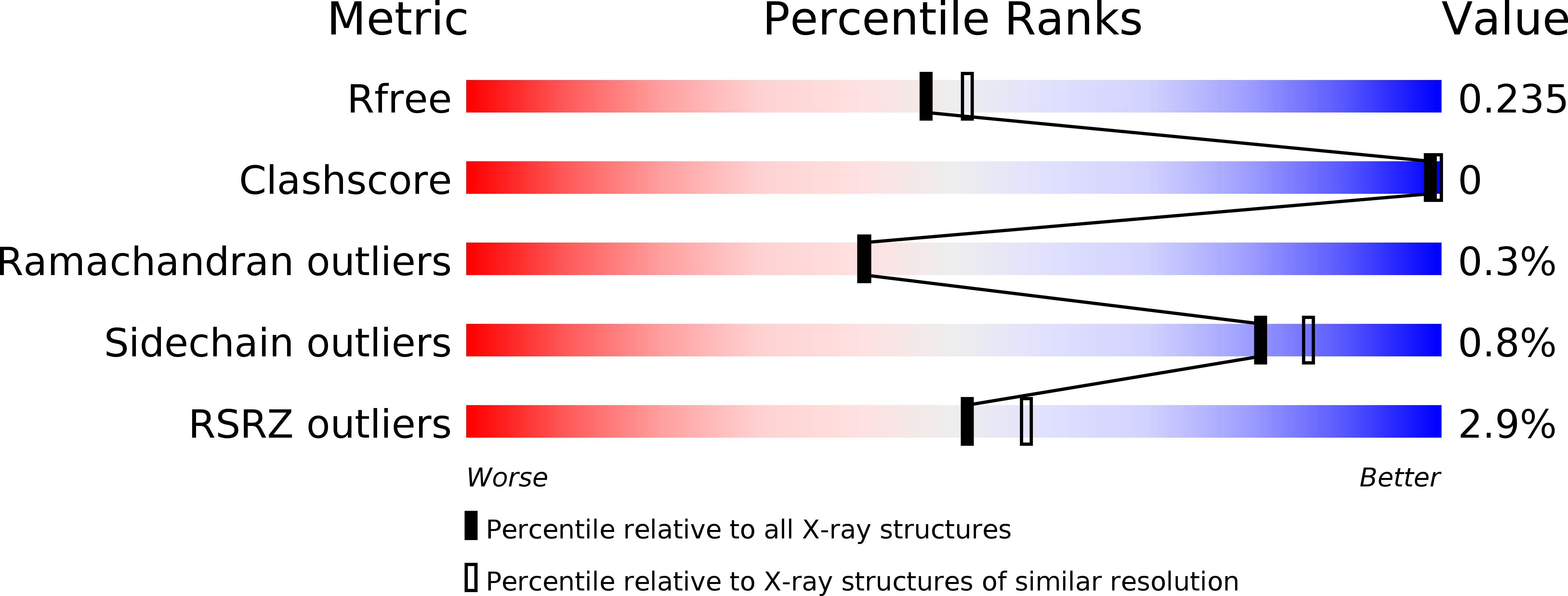
Resolution:
2.10 Å
R-Value Free:
0.23
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 2 21 21