Deposition Date
2017-08-21
Release Date
2017-11-29
Last Version Date
2024-10-09
Entry Detail
PDB ID:
6AR3
Keywords:
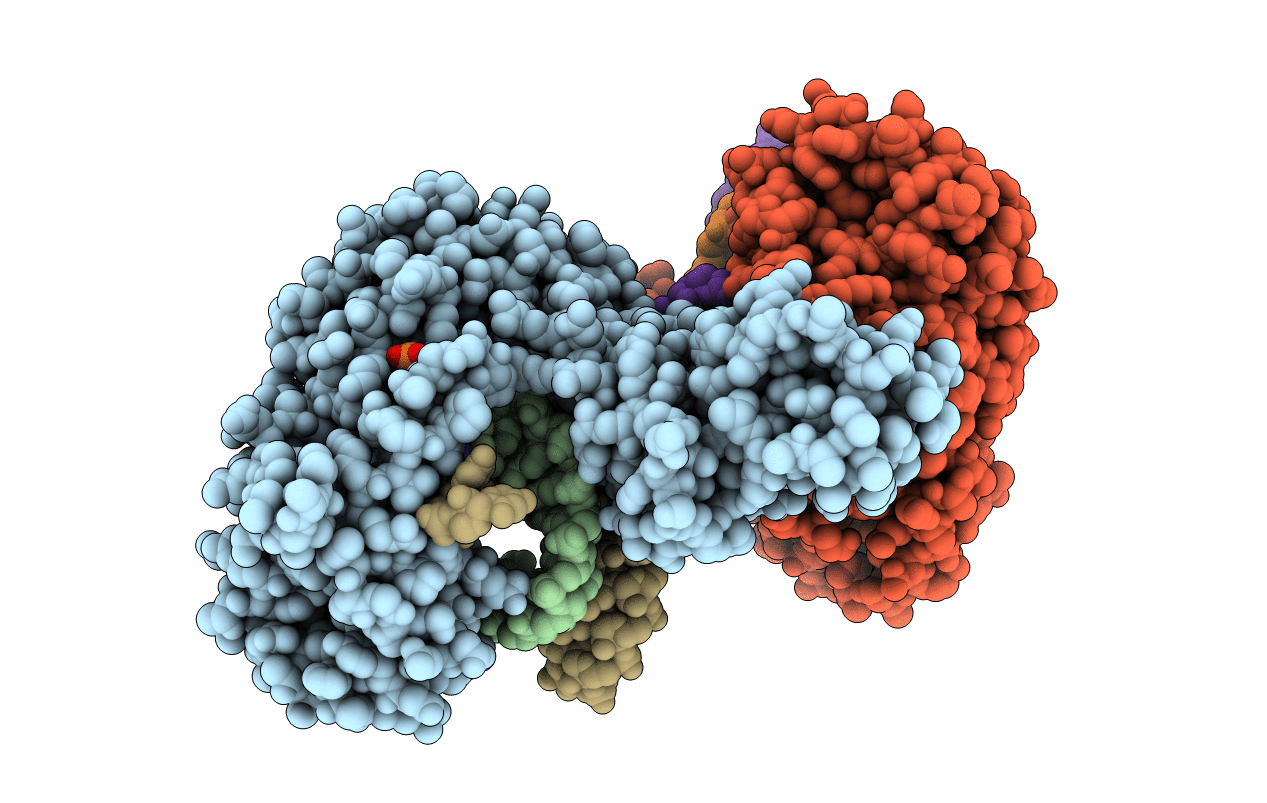
Title:
Structure of a Thermostable Group II Intron Reverse Transcriptase with Template-Primer and Its Functional and Evolutionary Implications (RT/Duplex (Se-Met))
Biological Source:
Source Organism(s):
Geobacillus stearothermophilus (Taxon ID: 1422)
synthetic construct (Taxon ID: 32630)
synthetic construct (Taxon ID: 32630)
Expression System(s):
Method Details:
Experimental Method:
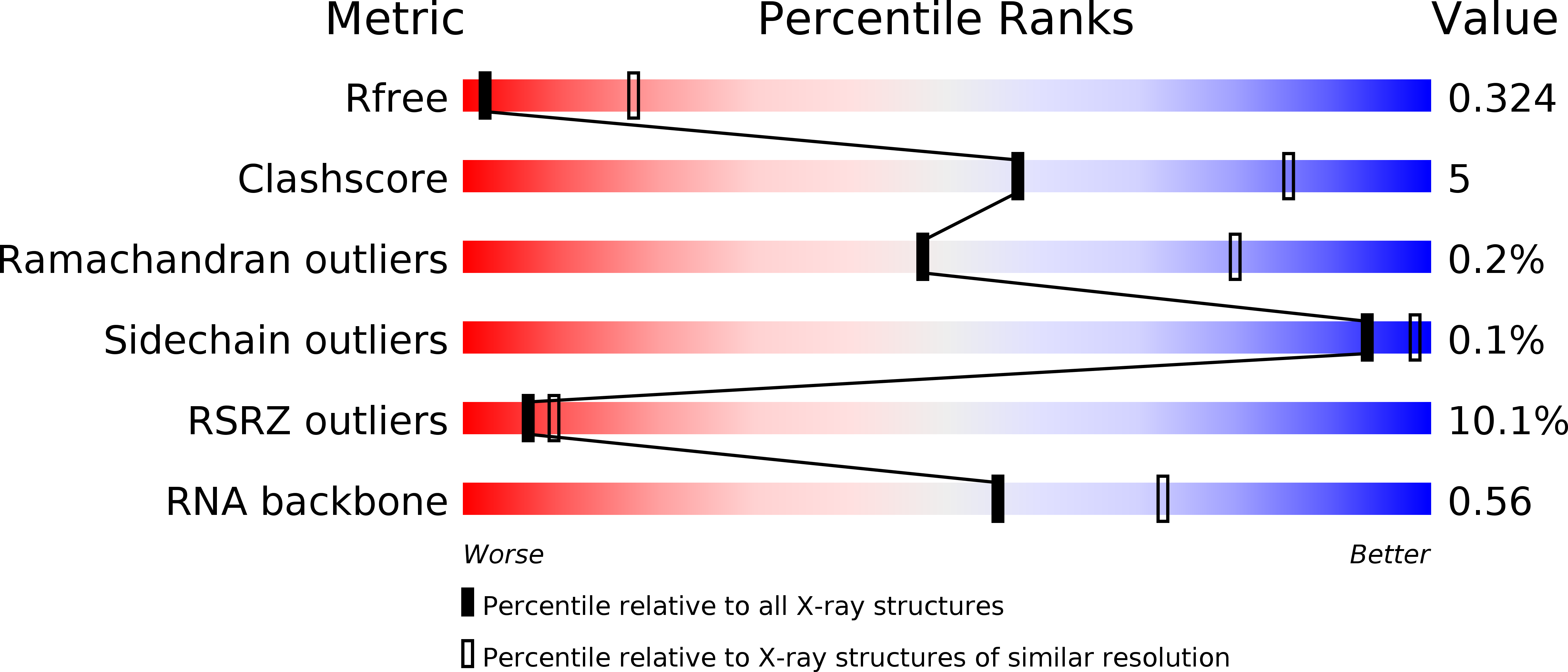
Resolution:
3.41 Å
R-Value Free:
0.32
R-Value Work:
0.27
R-Value Observed:
0.27
Space Group:
C 1 2 1