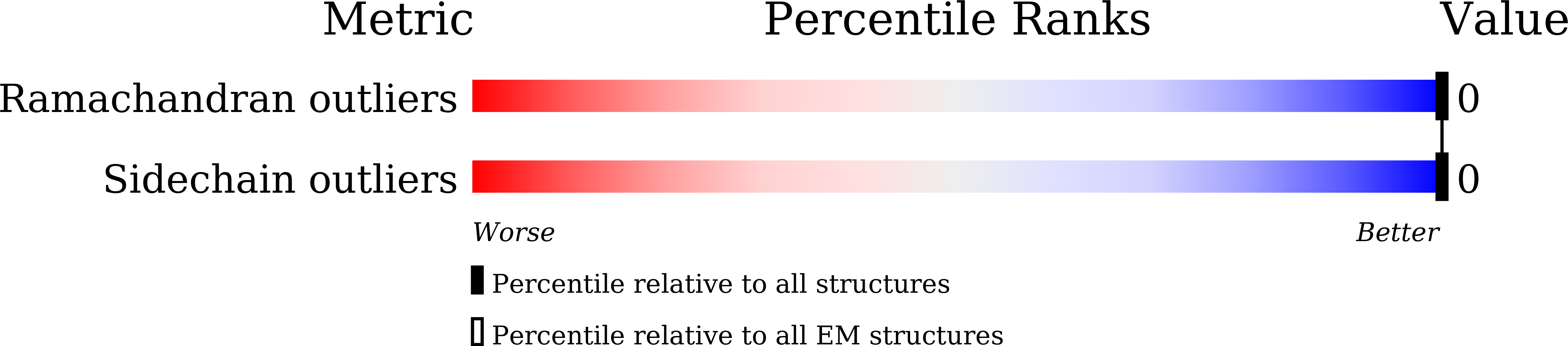
Deposition Date
2018-07-03
Release Date
2019-02-27
Last Version Date
2024-03-27
Entry Detail
PDB ID:
6A7F
Keywords:
Title:
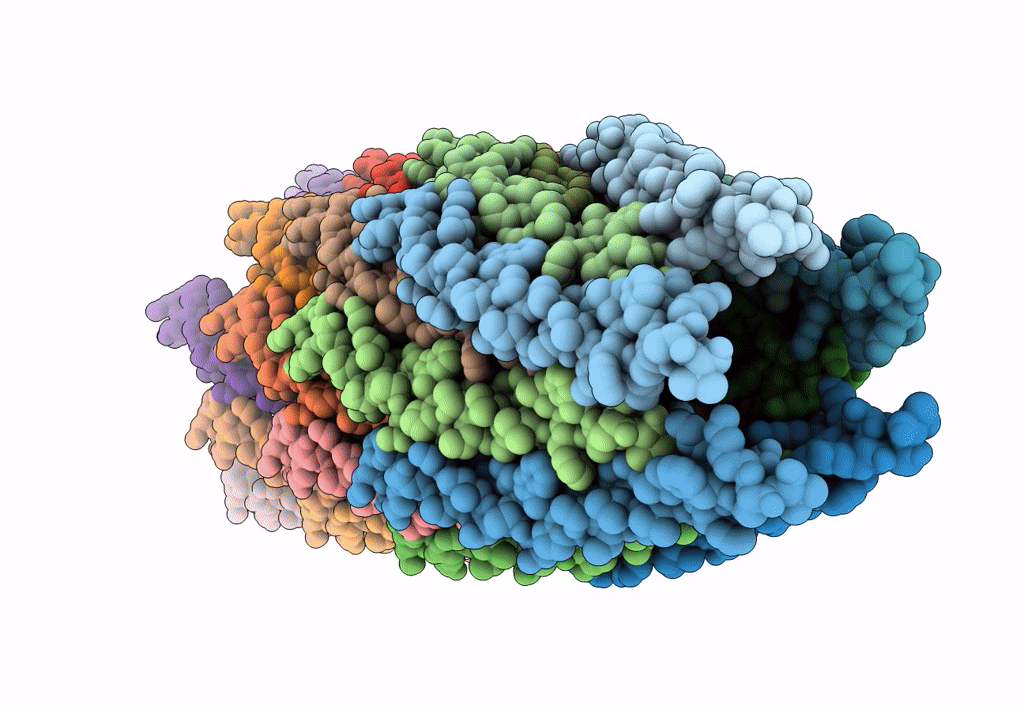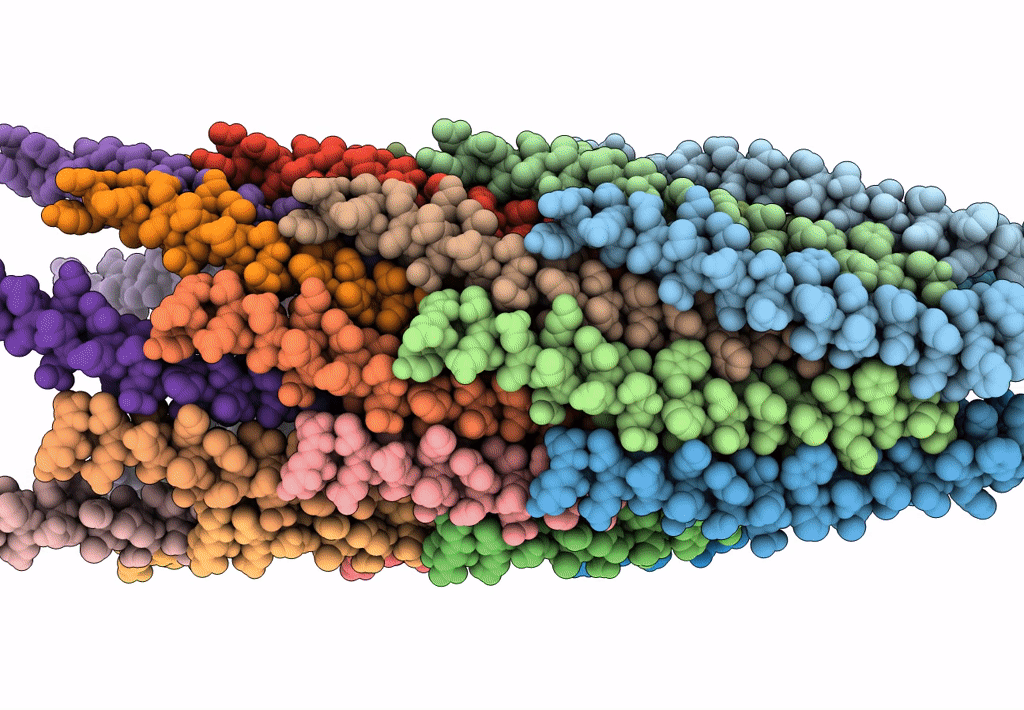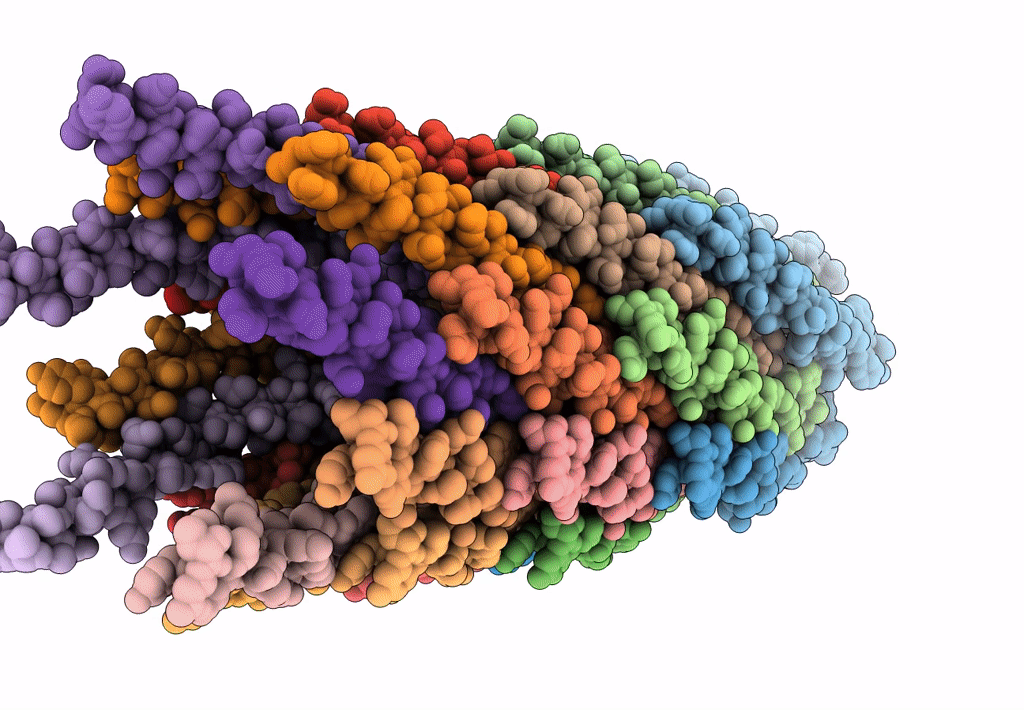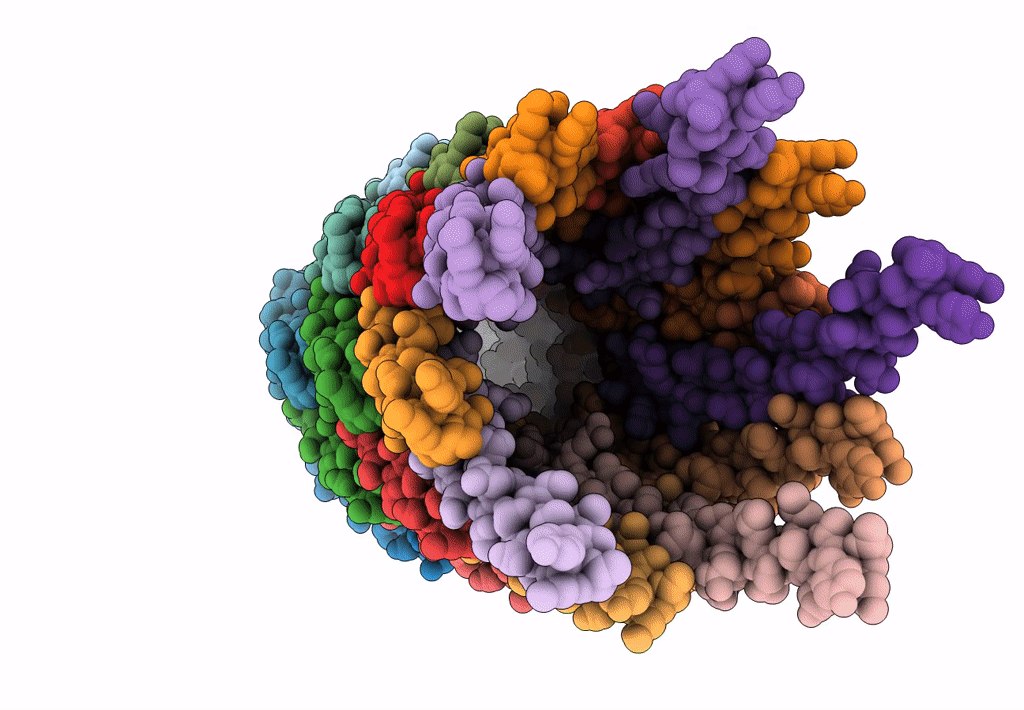
The cryo-EM structure of filamentous bacteriophage IKe major coat protein p8 shell assembly.
Biological Source:
Source Organism(s):
Filamentous phage (Taxon ID: 10867)
Method Details:
Experimental Method:
Resolution:
3.40 Å
Aggregation State:
FILAMENT
Reconstruction Method:
HELICAL