Deposition Date
2018-06-06
Release Date
2018-11-28
Last Version Date
2023-11-22
Entry Detail
PDB ID:
6A0U
Keywords:
Title:
Homoserine dehydrogenase K195A mutant from Thermus thermophilus HB8 complexed with HSE and NADP+
Biological Source:
Source Organism:
Thermus thermophilus HB8 (Taxon ID: 300852)
Host Organism:
Method Details:
Experimental Method:
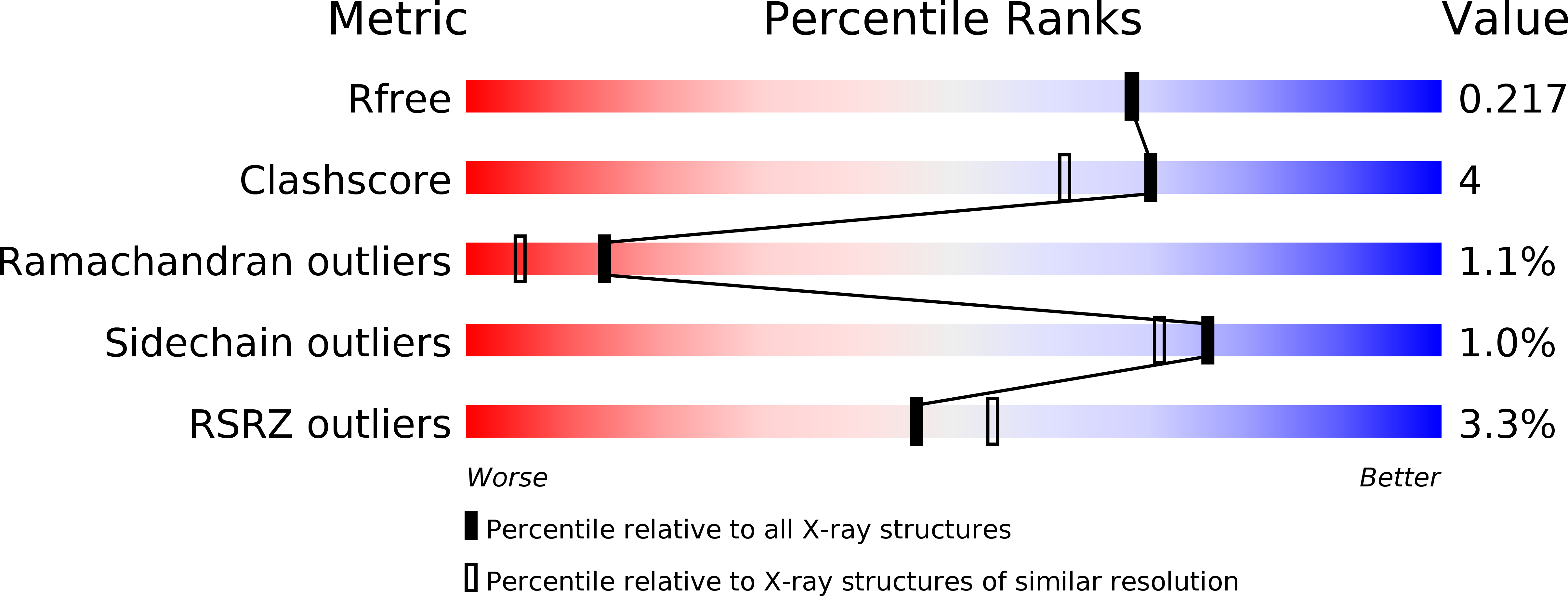
Resolution:
1.93 Å
R-Value Free:
0.21
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 32 2 1