Deposition Date
2018-01-31
Release Date
2018-08-15
Last Version Date
2023-11-22
Entry Detail
PDB ID:
5Z86
Keywords:
Title:
azide-bound cytochrome c oxidase structure determined using the crystals exposed to 20 mM azide solution for 3 days
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Method Details:
Experimental Method:
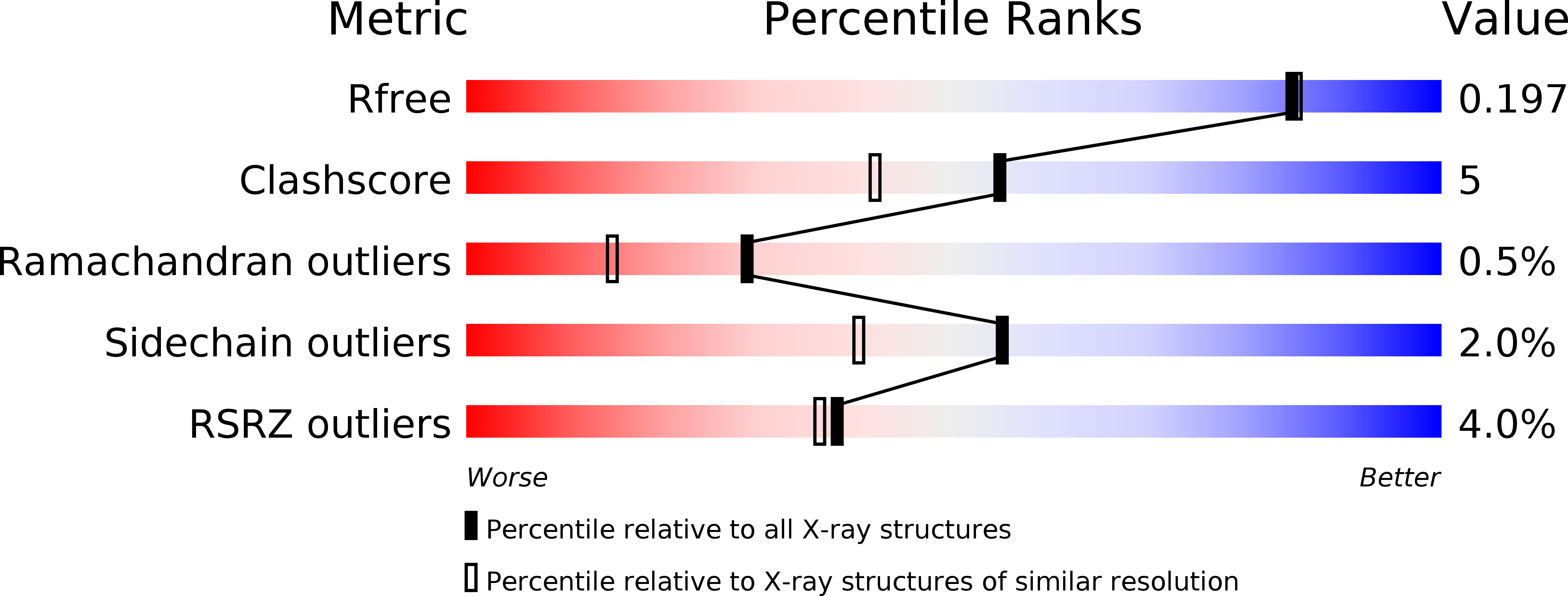
Resolution:
1.85 Å
R-Value Free:
0.19
R-Value Work:
0.18
R-Value Observed:
0.18
Space Group:
P 21 21 21