Deposition Date
2018-01-02
Release Date
2018-10-03
Last Version Date
2023-11-22
Entry Detail
PDB ID:
5Z2K
Keywords:
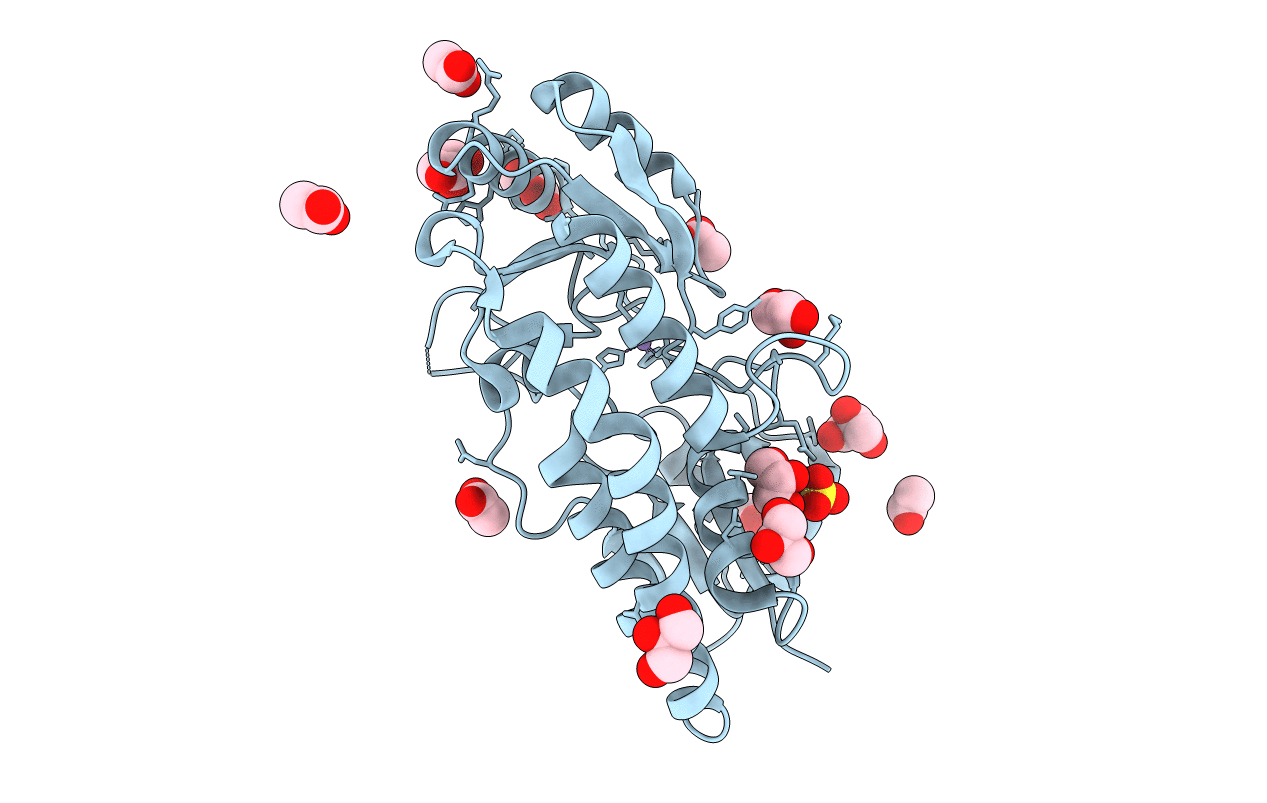
Title:
Structure of S38A mutant Mn-bound periplasmic metal binding protein from candidatus liberibacter asiaticus
Biological Source:
Source Organism(s):
Liberibacter asiaticus (strain psy62) (Taxon ID: 537021)
Expression System(s):
Method Details:
Experimental Method:
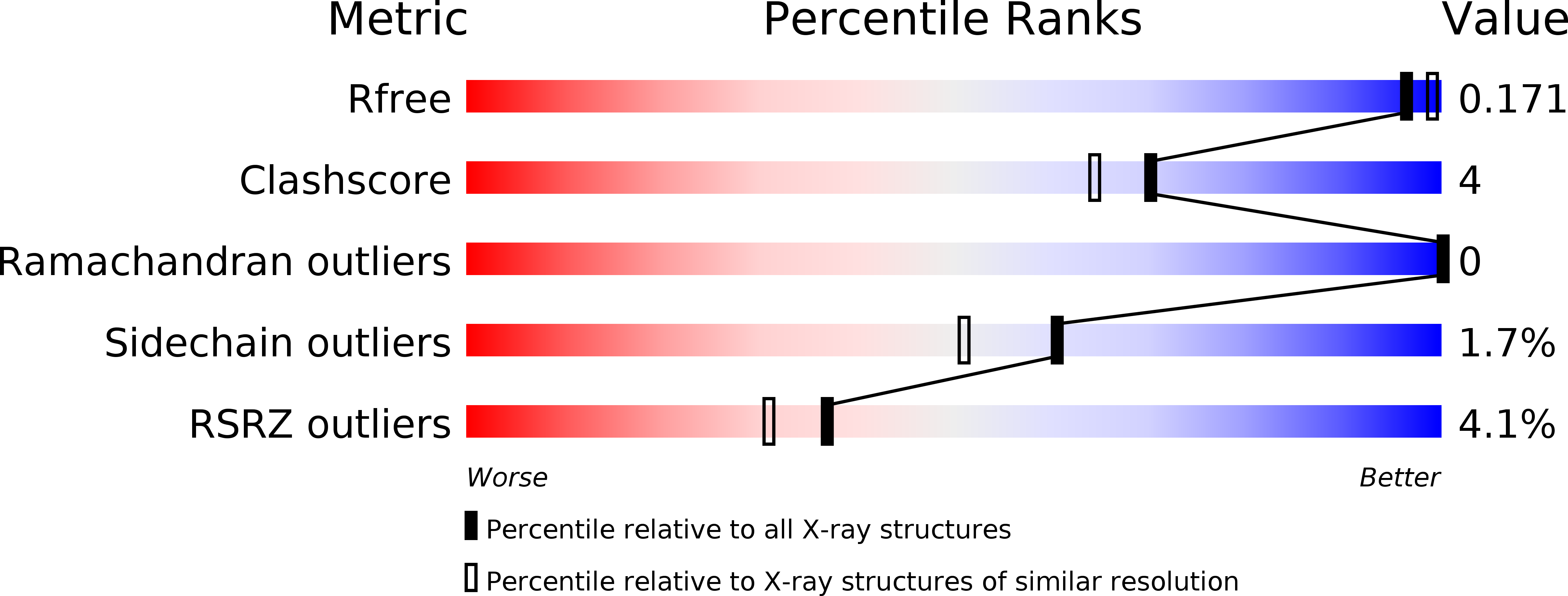
Resolution:
1.80 Å
R-Value Free:
0.16
R-Value Work:
0.14
R-Value Observed:
0.14
Space Group:
P 32 2 1