Deposition Date
2017-12-28
Release Date
2018-03-07
Last Version Date
2024-10-30
Entry Detail
PDB ID:
5Z25
Keywords:
Title:
Trimeric Alpha-Helix-Inserted Circular Permutant of Cytochrome c555
Biological Source:
Source Organism(s):
Aquifex aeolicus (strain VF5) (Taxon ID: 224324)
Expression System(s):
Method Details:
Experimental Method:
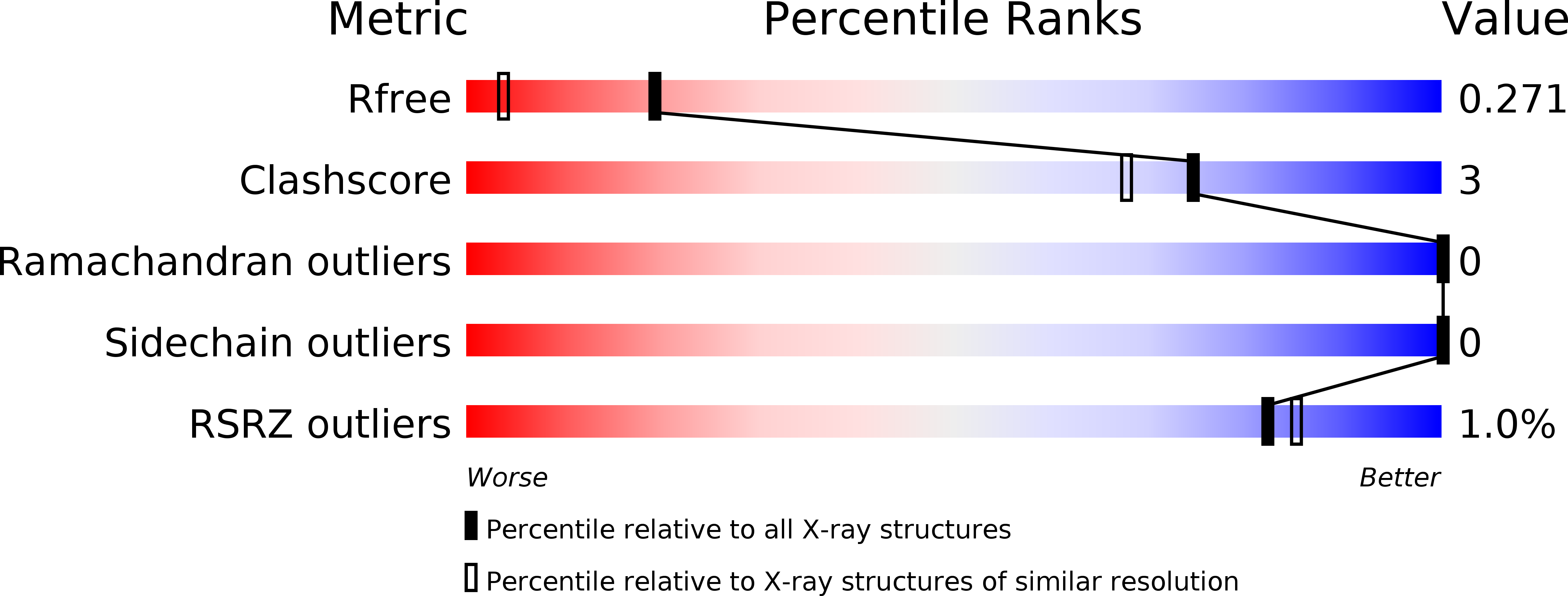
Resolution:
1.70 Å
R-Value Free:
0.26
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
F 4 3 2