Deposition Date
2017-12-15
Release Date
2018-10-03
Last Version Date
2024-03-27
Entry Detail
PDB ID:
5YZV
Keywords:
Title:
Biophysical and structural characterization of the thermostable WD40 domain of a prokaryotic protein, Thermomonospora curvata PkwA
Biological Source:
Source Organism:
Thermomonospora curvata (Taxon ID: 2020)
Host Organism:
Method Details:
Experimental Method:
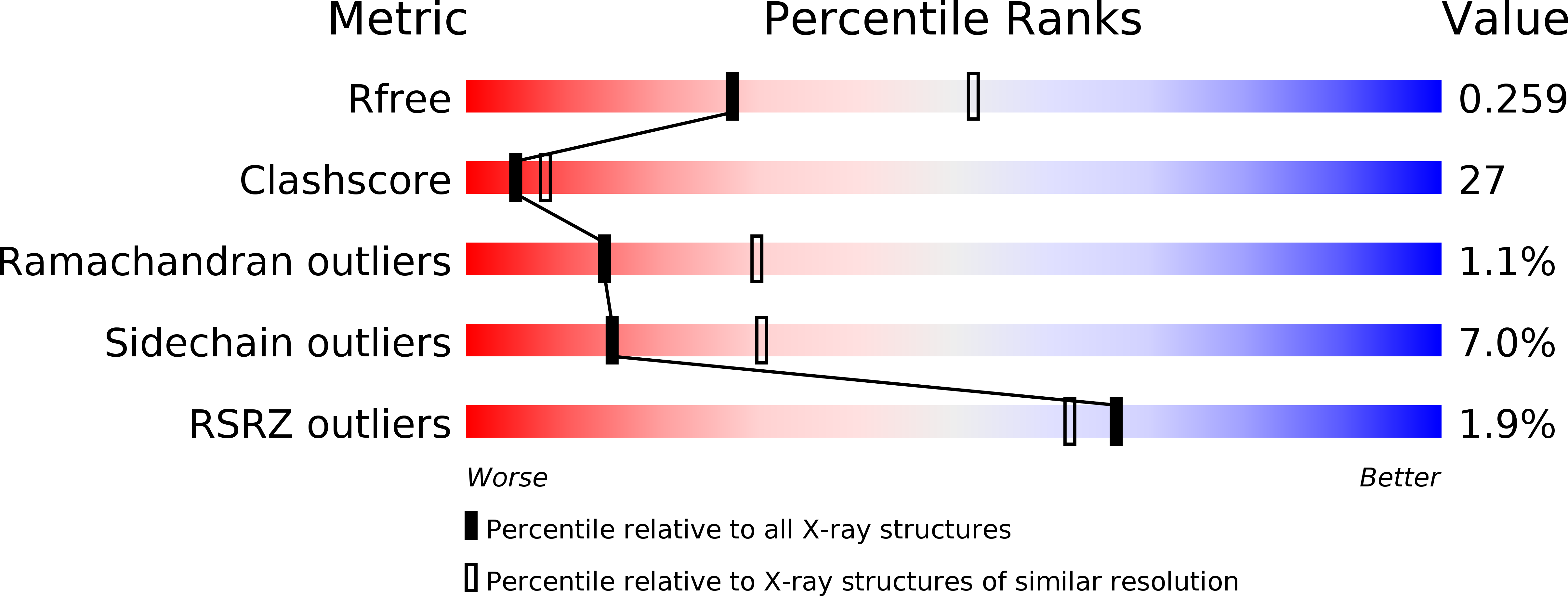
Resolution:
2.60 Å
R-Value Free:
0.25
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
P 1