Deposition Date
2017-11-13
Release Date
2018-10-17
Last Version Date
2023-11-22
Entry Detail
PDB ID:
5YS5
Keywords:
Title:
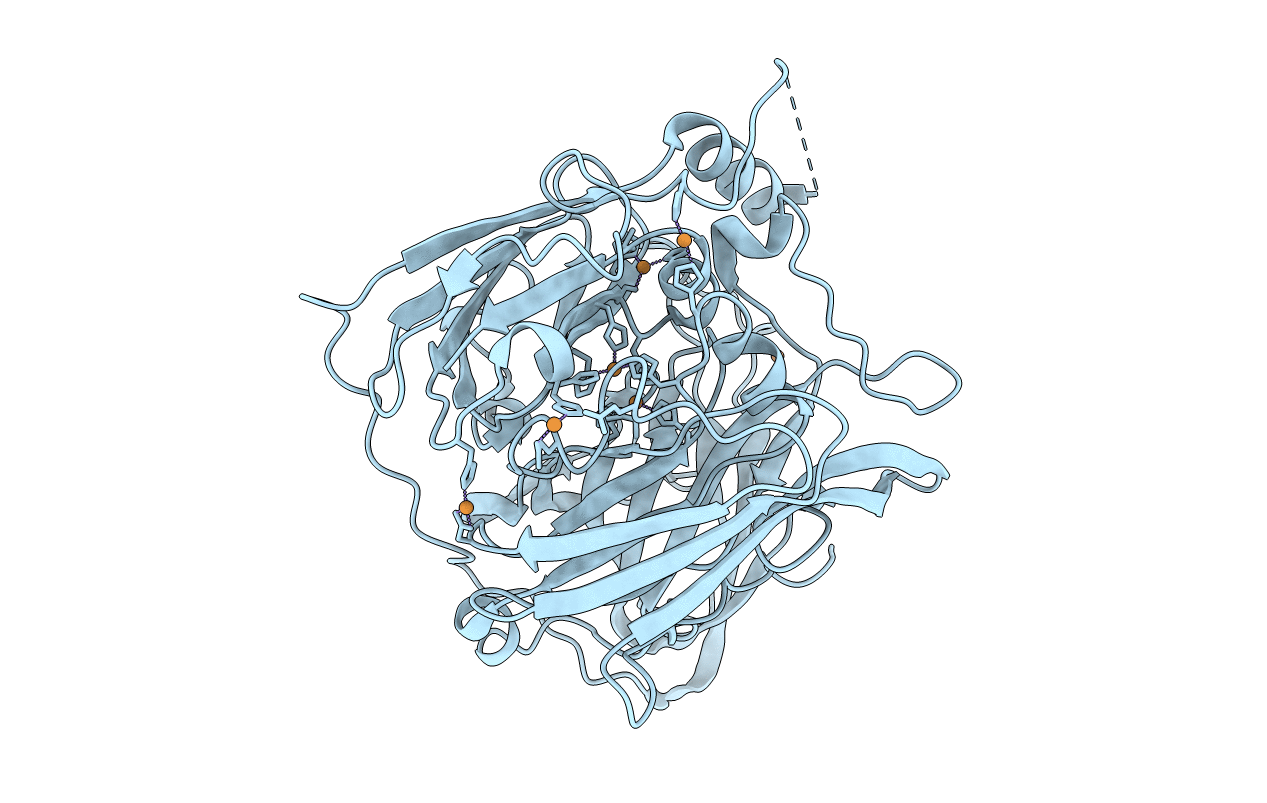
Crystal structure of Multicopper Oxidase CueO G304K mutant with seven copper ions
Biological Source:
Source Organism(s):
Escherichia coli K12 (Taxon ID: 83333)
Expression System(s):
Method Details:
Experimental Method:
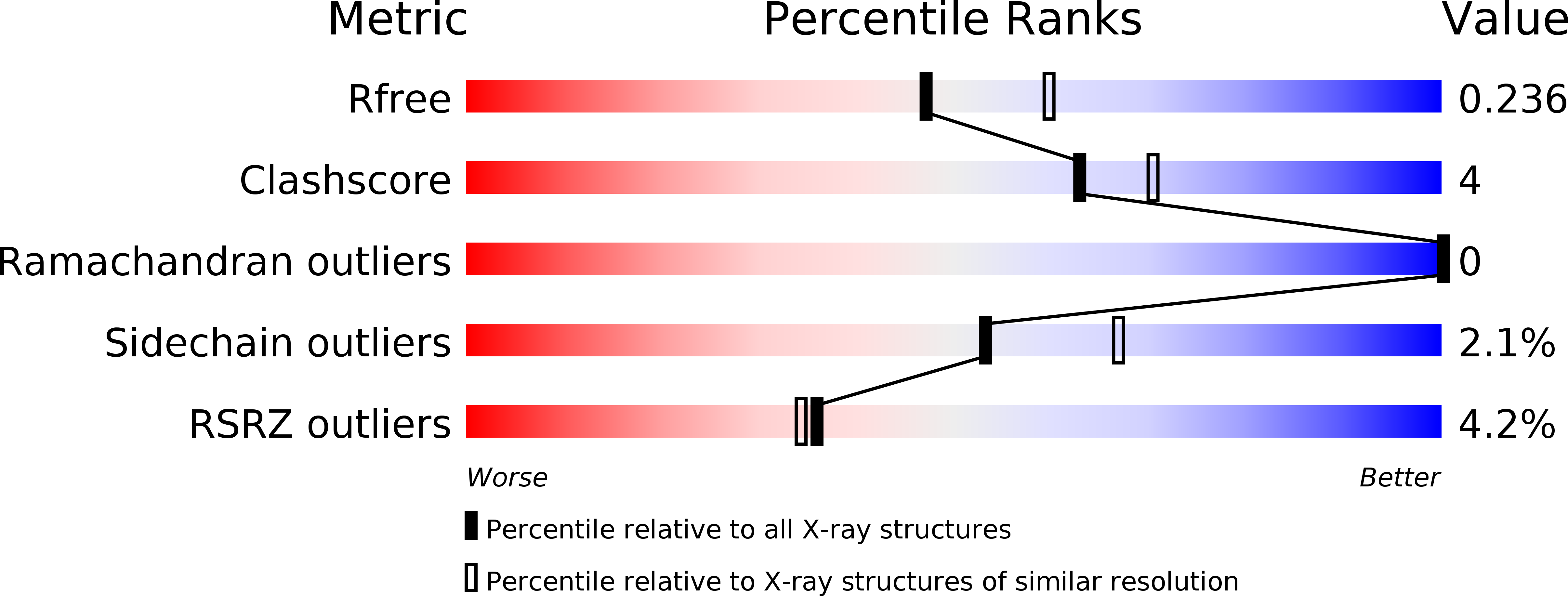
Resolution:
2.20 Å
R-Value Free:
0.23
R-Value Work:
0.18
R-Value Observed:
0.19
Space Group:
P 1 21 1