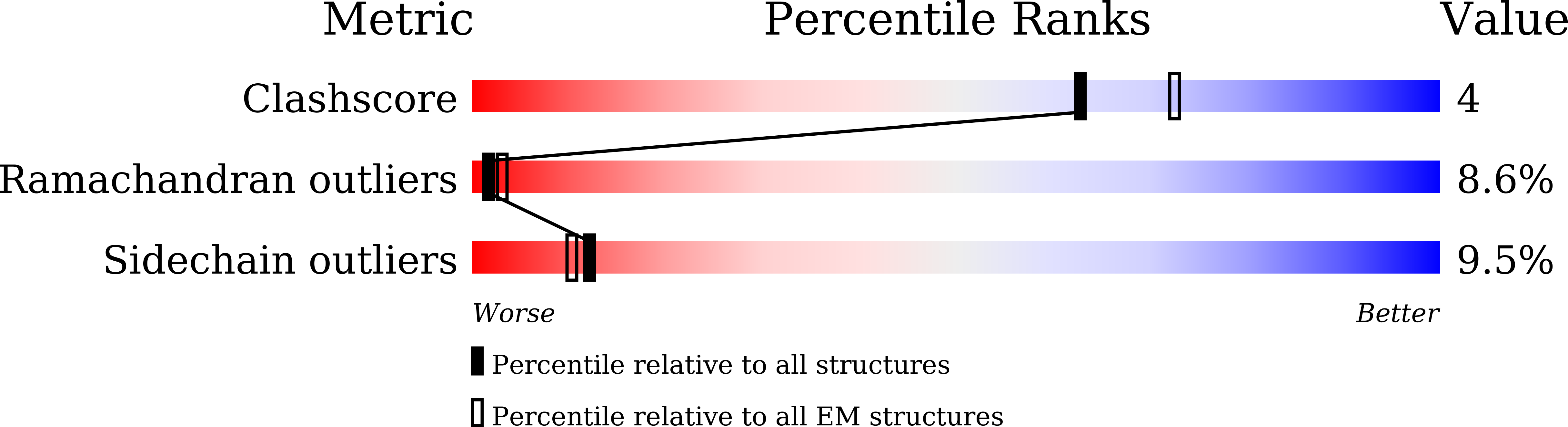
Deposition Date
2017-08-10
Release Date
2018-01-24
Last Version Date
2024-11-13
Entry Detail
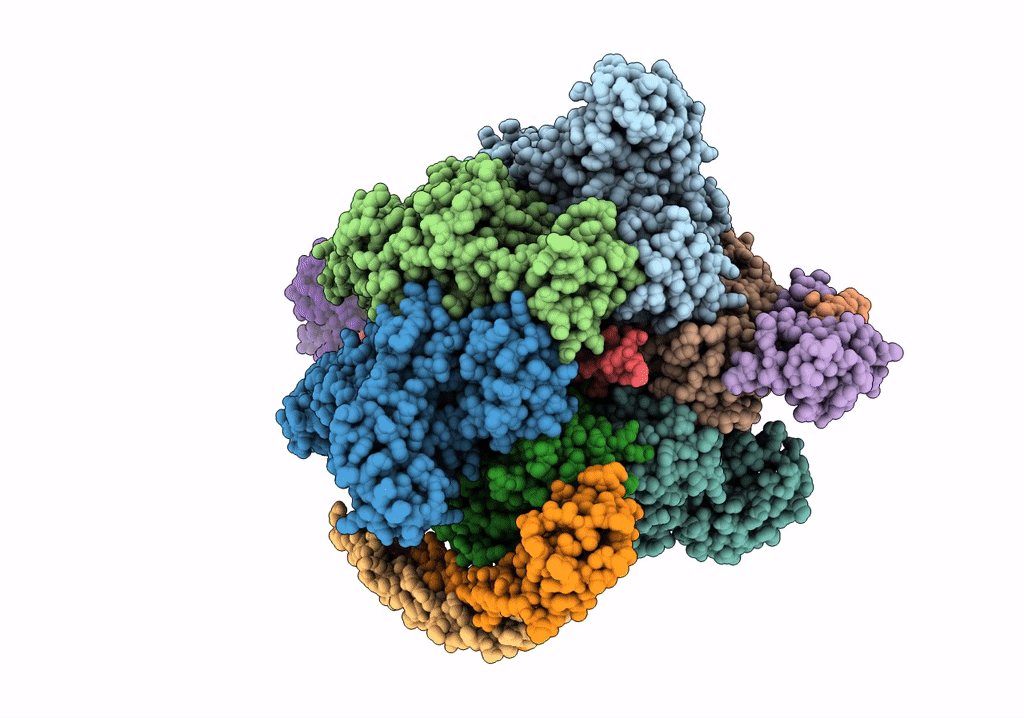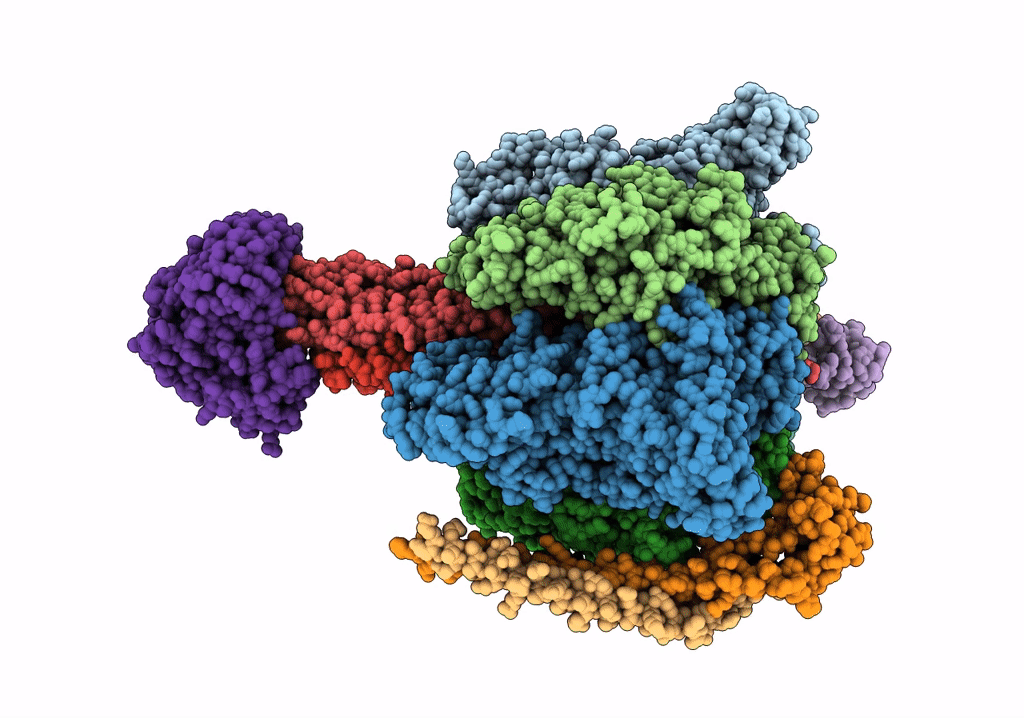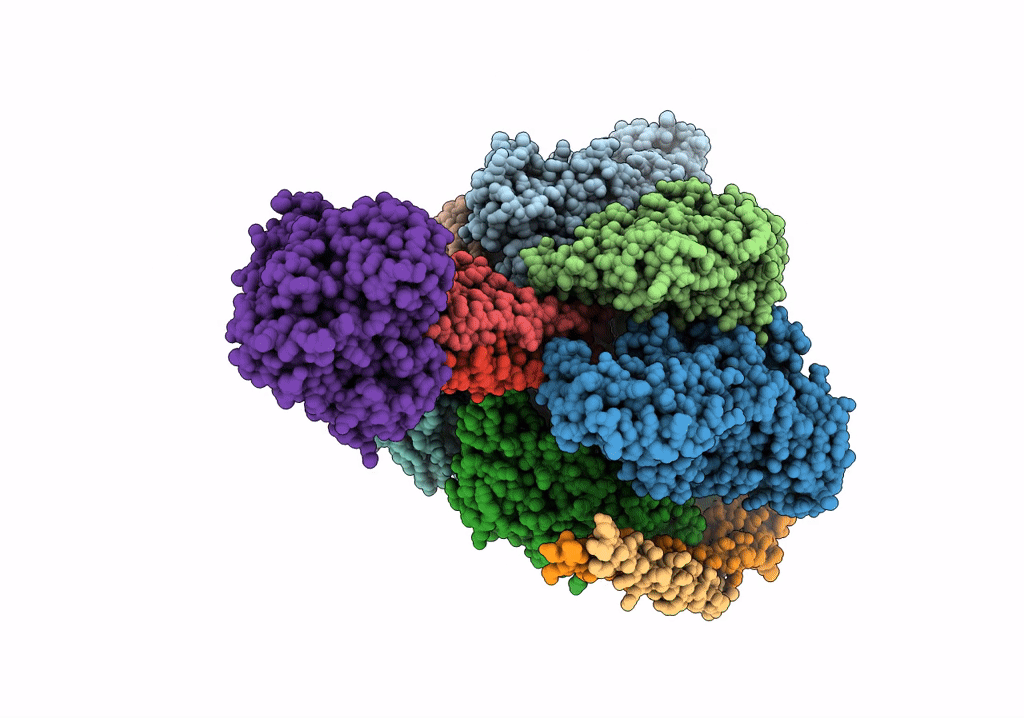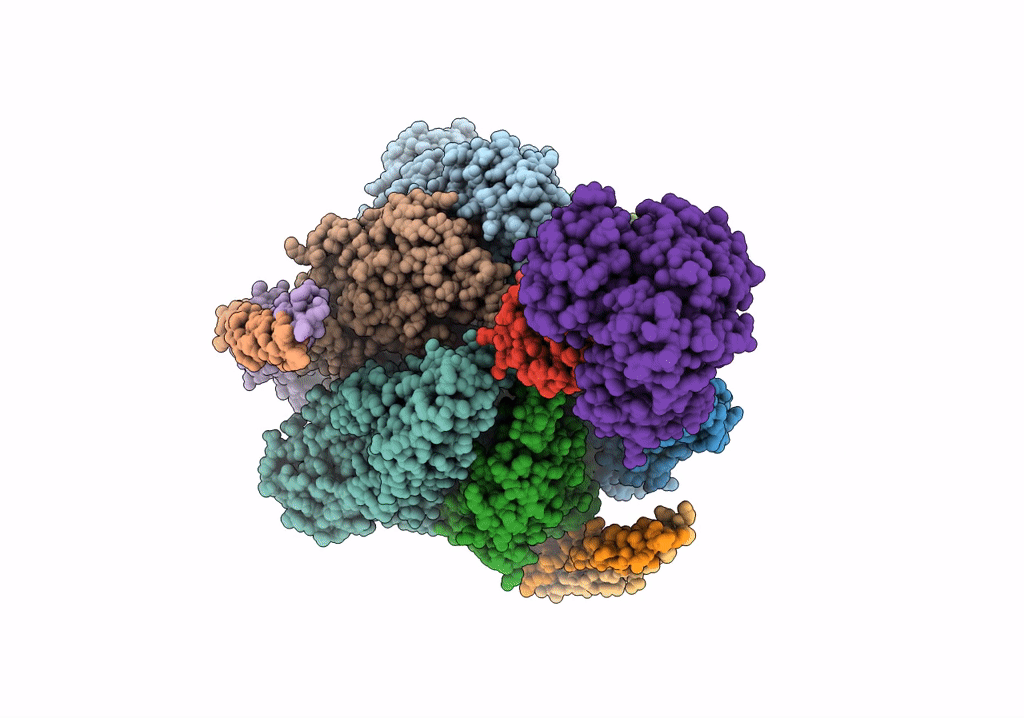
PDB ID:
5Y5Y
Keywords:
Title:
V/A-type ATPase/synthase from Thermus thermophilus, peripheral domain, rotational state 1
Biological Source:
Source Organism(s):
Thermus thermophilus HB8 (Taxon ID: 300852)
Method Details:
Experimental Method:
Resolution:
4.70 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE