Deposition Date
2017-06-29
Release Date
2018-03-28
Last Version Date
2024-10-16
Entry Detail
PDB ID:
5XW8
Keywords:
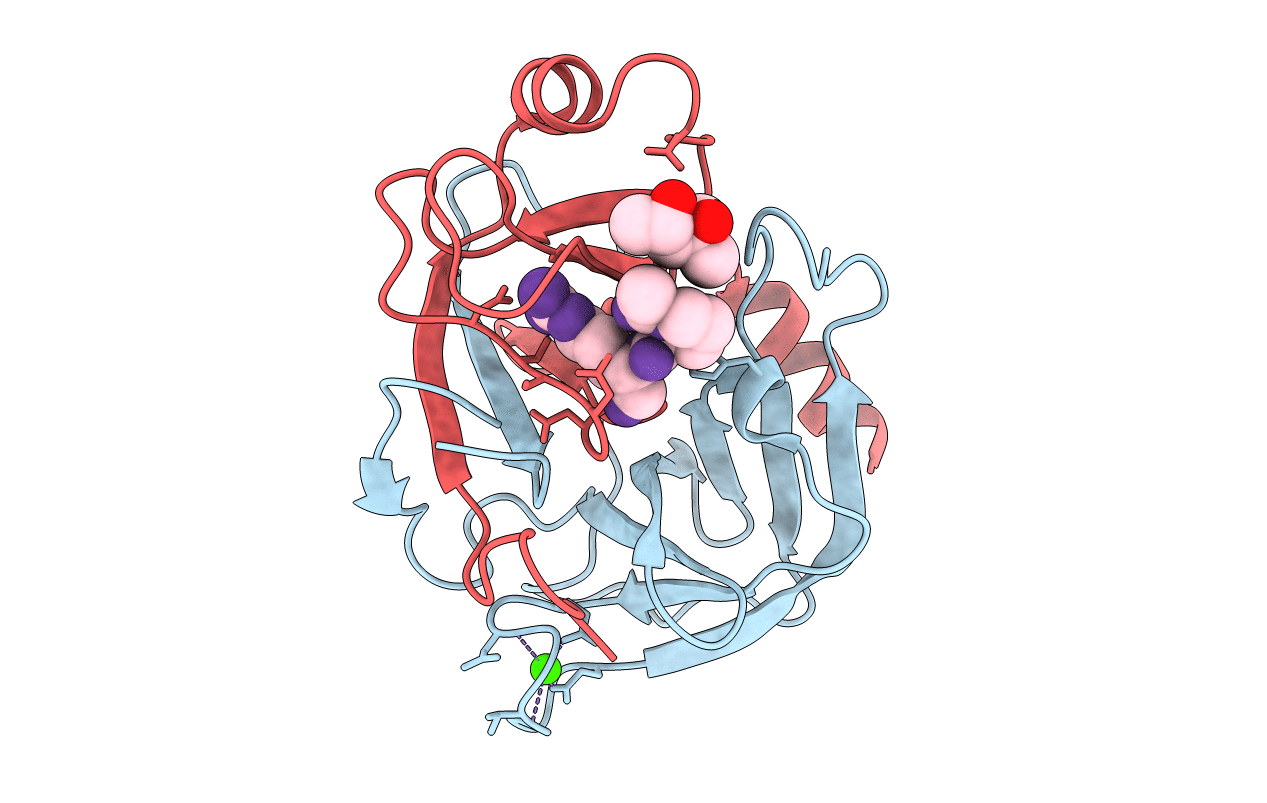
Title:
Crystal Structure of Porcine pancreatic trypsin with tripeptide inhibitor, PRN, at pH 7
Biological Source:
Source Organism(s):
Capsicum annuum (Taxon ID: 4072)
Sus scrofa (Taxon ID: 9823)
Sus scrofa (Taxon ID: 9823)
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Free:
0.17
R-Value Work:
0.14
R-Value Observed:
0.15
Space Group:
P 61 2 2


