Deposition Date
2017-05-11
Release Date
2018-01-10
Last Version Date
2024-03-27
Entry Detail
PDB ID:
5XLP
Keywords:
Title:
Anti-CRISPR proteins AcrF1/2 bound to Csy surveillance complex with a 20nt spacer crRNA backbone region
Biological Source:
Source Organism(s):
Pseudomonas aeruginosa (strain UCBPP-PA14) (Taxon ID: 208963)
Pseudomonas aeruginosa (Taxon ID: 287)
Pseudomonas phage JBD30 (Taxon ID: 1223260)
Pseudomonas aeruginosa (Taxon ID: 287)
Pseudomonas phage JBD30 (Taxon ID: 1223260)
Expression System(s):
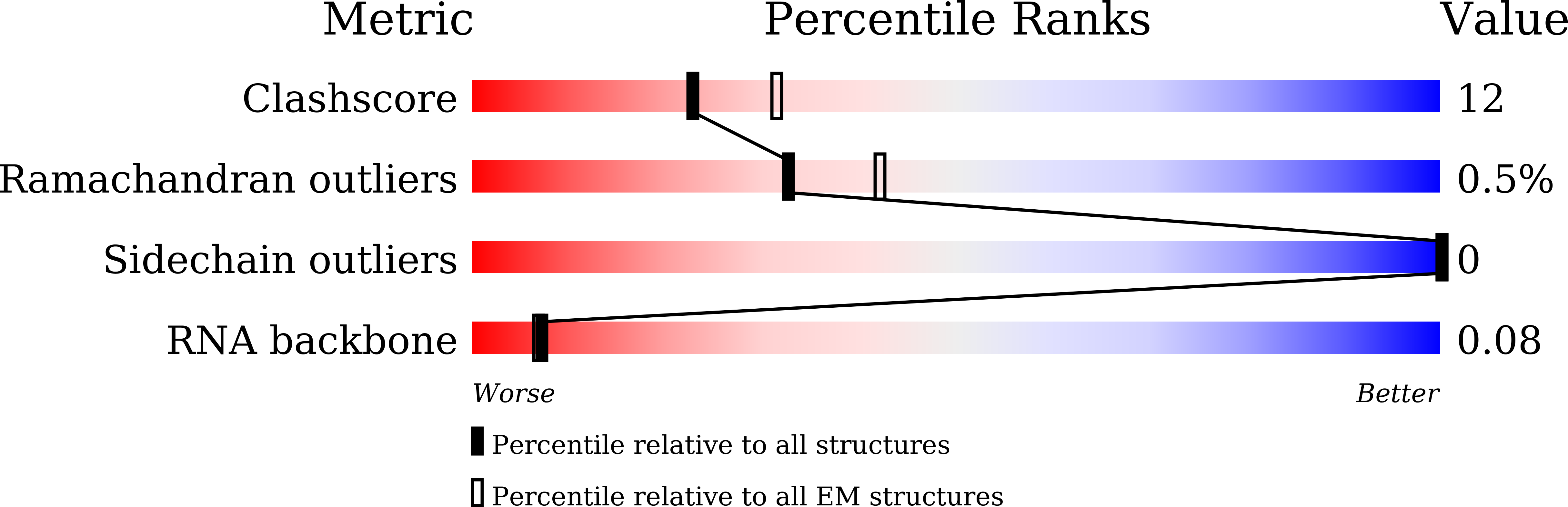
Method Details:
Experimental Method:
Resolution:
4.20 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE