Deposition Date
2017-03-11
Release Date
2017-06-14
Last Version Date
2024-10-30
Entry Detail
PDB ID:
5XA9
Keywords:
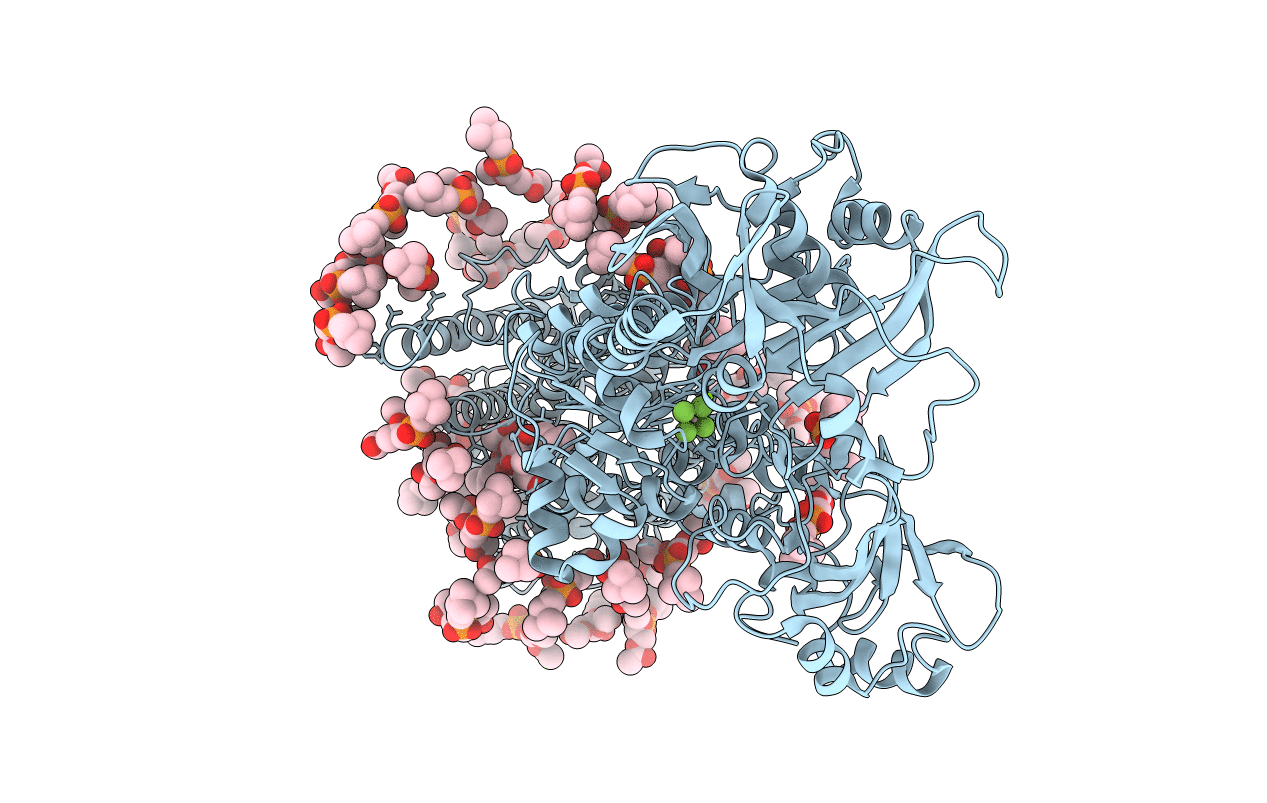
Title:
Complete structure factors and an atomic model of the calcium pump (SERCA1A) and associated phospholipids in the E2-ALF-(TG) crystals of C2 symmetry
Biological Source:
Source Organism(s):
Oryctolagus cuniculus (Taxon ID: 9986)
Method Details:
Experimental Method:
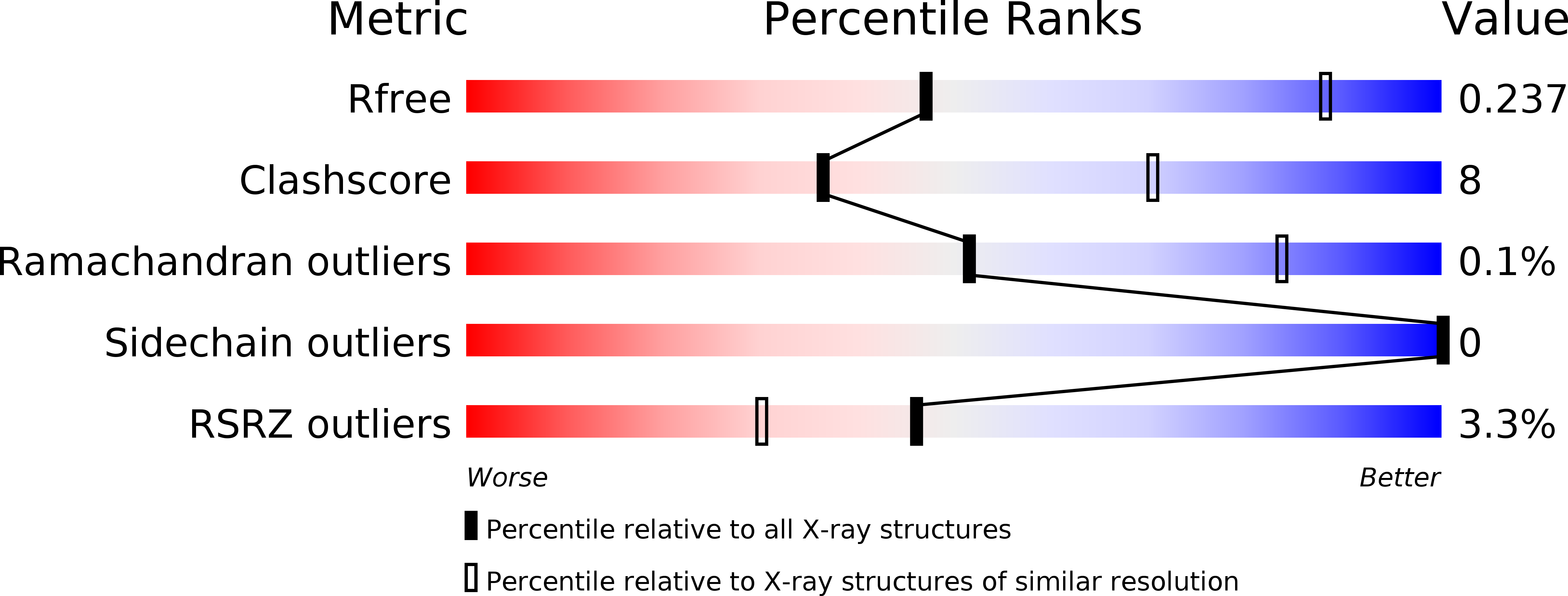
Resolution:
3.20 Å
R-Value Free:
0.23
R-Value Work:
0.21
R-Value Observed:
0.21
Space Group:
C 1 2 1