Deposition Date
2017-03-02
Release Date
2017-06-07
Last Version Date
2023-11-22
Entry Detail
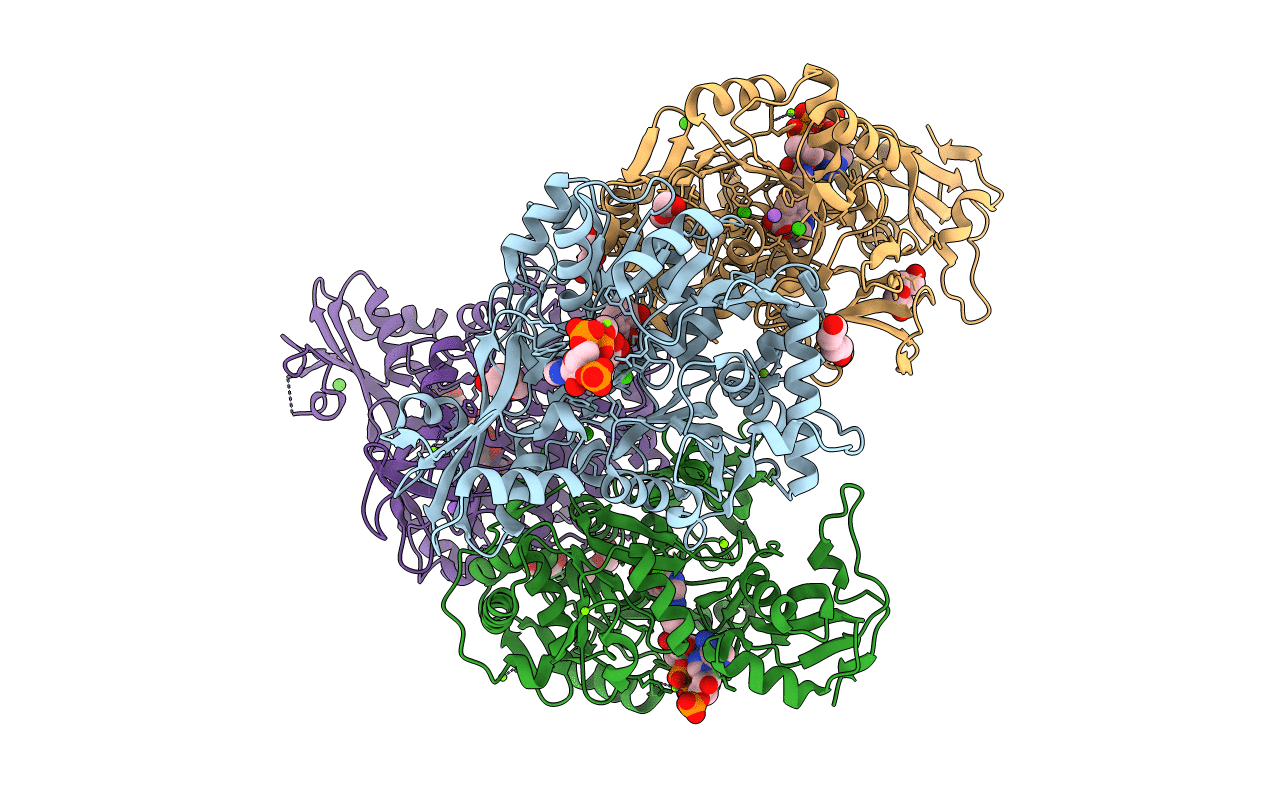
PDB ID:
5X8G
Keywords:
Title:
Binary complex structure of a double mutant I454RA456K of o-Succinylbenzoate CoA Synthetase (MenE) from Bacillus Subtilis bound with its product analogue OSB-NCoA at 1.90 angstrom
Biological Source:
Source Organism(s):
Bacillus subtilis subsp. subtilis str. 168 (Taxon ID: 224308)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
1.90 Å
R-Value Free:
0.20
R-Value Work:
0.16
R-Value Observed:
0.16
Space Group:
P 1


