Deposition Date
2016-12-29
Release Date
2018-06-13
Last Version Date
2024-11-06
Entry Detail
PDB ID:
5WVX
Keywords:
Title:
Crystal Structure of bifunctional Kunitz type Trypsin /amylase inhibitor (AMTIN) from the tubers of Alocasia macrorrhiza
Biological Source:
Source Organism(s):
Alocasia macrorrhizos (Taxon ID: 4456)
Method Details:
Experimental Method:
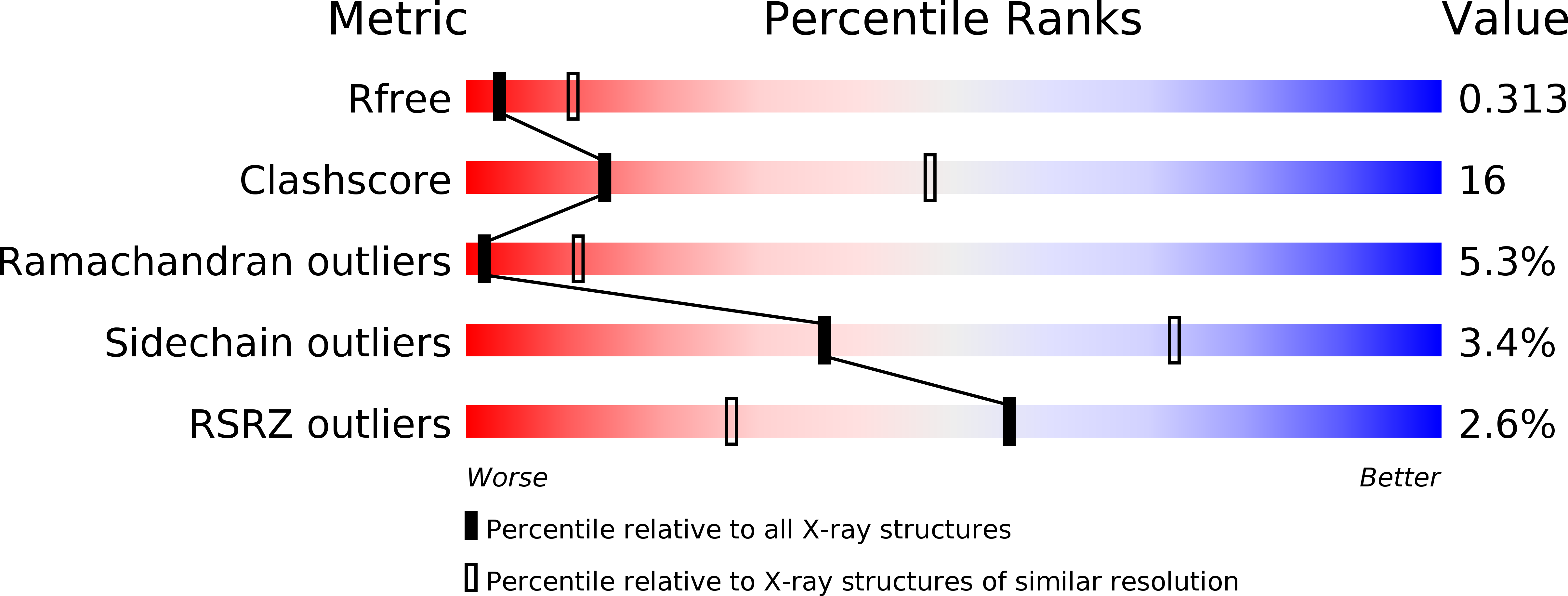
Resolution:
3.00 Å
R-Value Free:
0.31
R-Value Work:
0.27
R-Value Observed:
0.29
Space Group:
P 41