Deposition Date
2016-12-05
Release Date
2017-12-06
Last Version Date
2023-11-08
Entry Detail
PDB ID:
5WS2
Keywords:
Title:
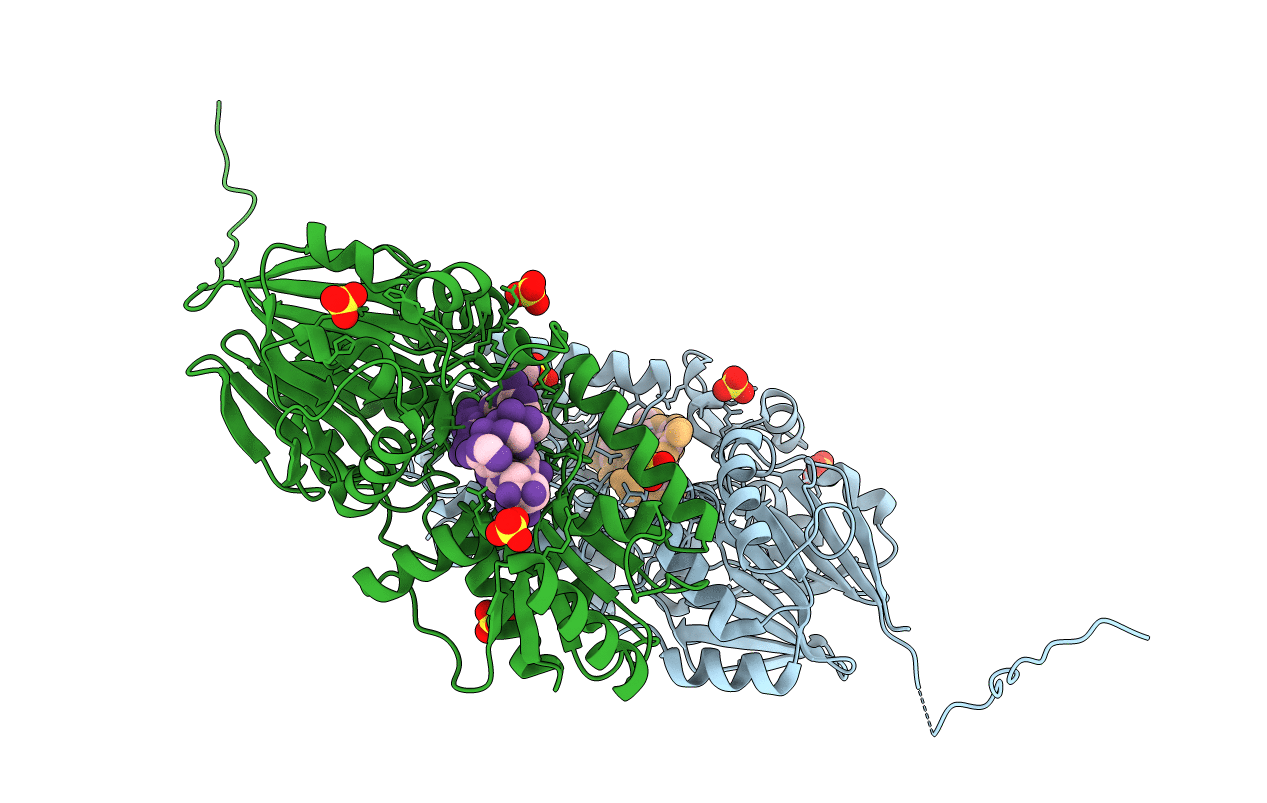
Crystal structure of mpy-RNase J (mutant S247A), an archaeal RNase J from Methanolobus psychrophilus R15, complex with RNA
Biological Source:
Source Organism(s):
Methanolobus psychrophilus R15 (Taxon ID: 1094980)
unidentified (Taxon ID: 32644)
unidentified (Taxon ID: 32644)
Expression System(s):
Method Details:
Experimental Method:
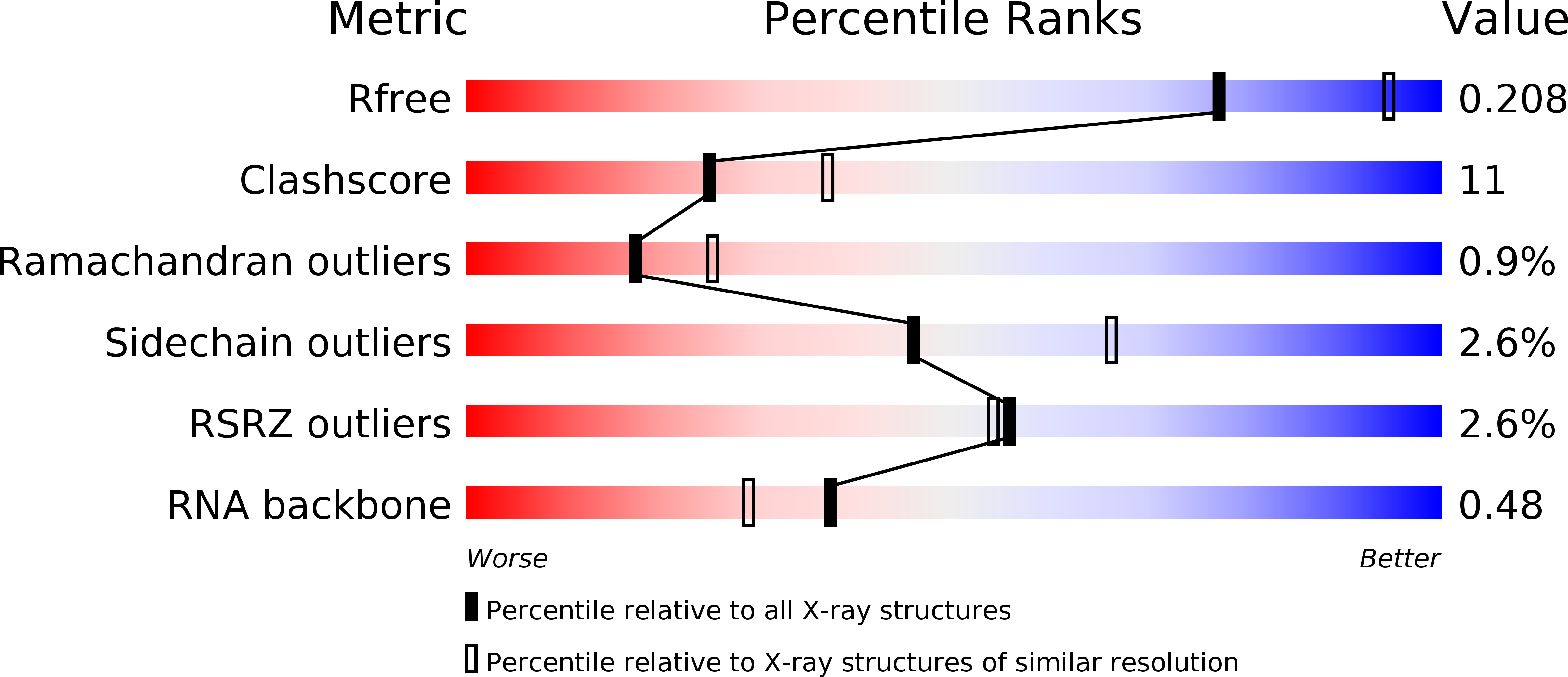
Resolution:
2.40 Å
R-Value Free:
0.20
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 65 2 2