Deposition Date
2017-06-30
Release Date
2017-07-19
Last Version Date
2024-10-30
Entry Detail
PDB ID:
5WCM
Keywords:
Title:
Crystal structure of the complex between class B3 beta-lactamase BJP-1 and 4-nitrobenzene-sulfonamide - new refinement
Biological Source:
Source Organism(s):
Expression System(s):
Method Details:
Experimental Method:
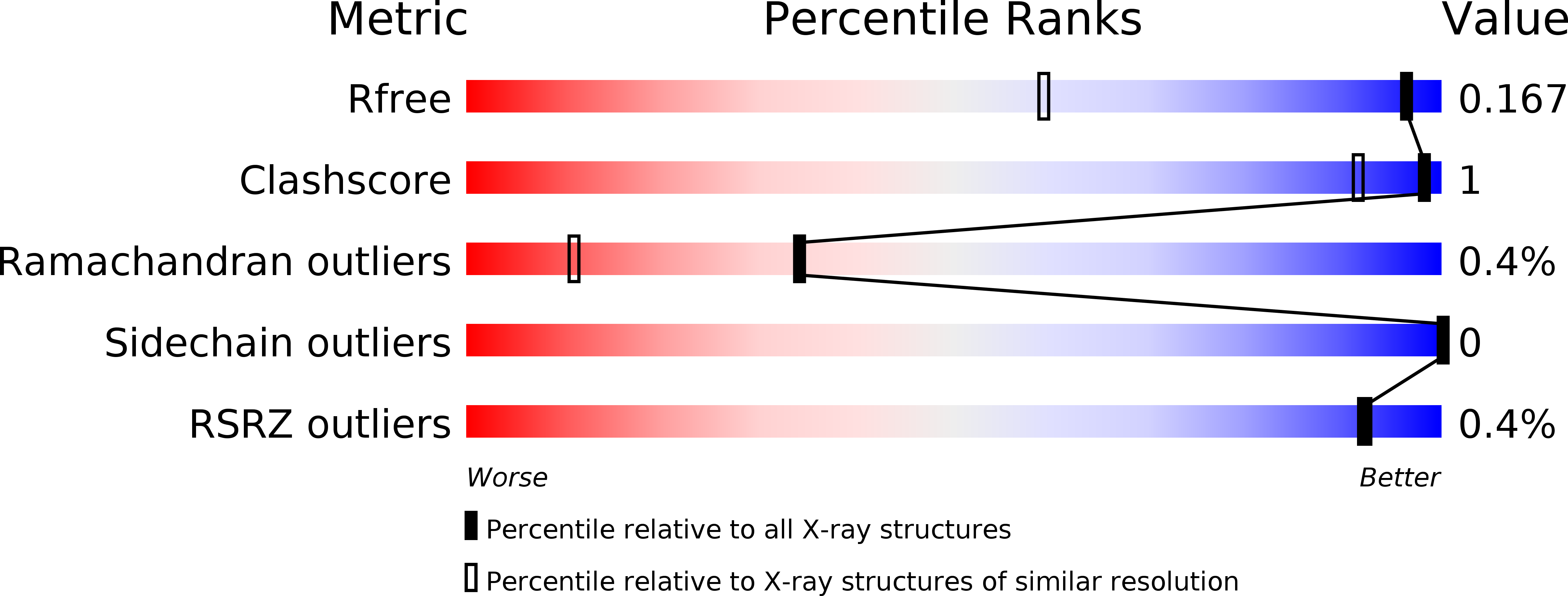
Resolution:
1.20 Å
R-Value Free:
0.15
R-Value Work:
0.13
R-Value Observed:
0.13
Space Group:
P 1