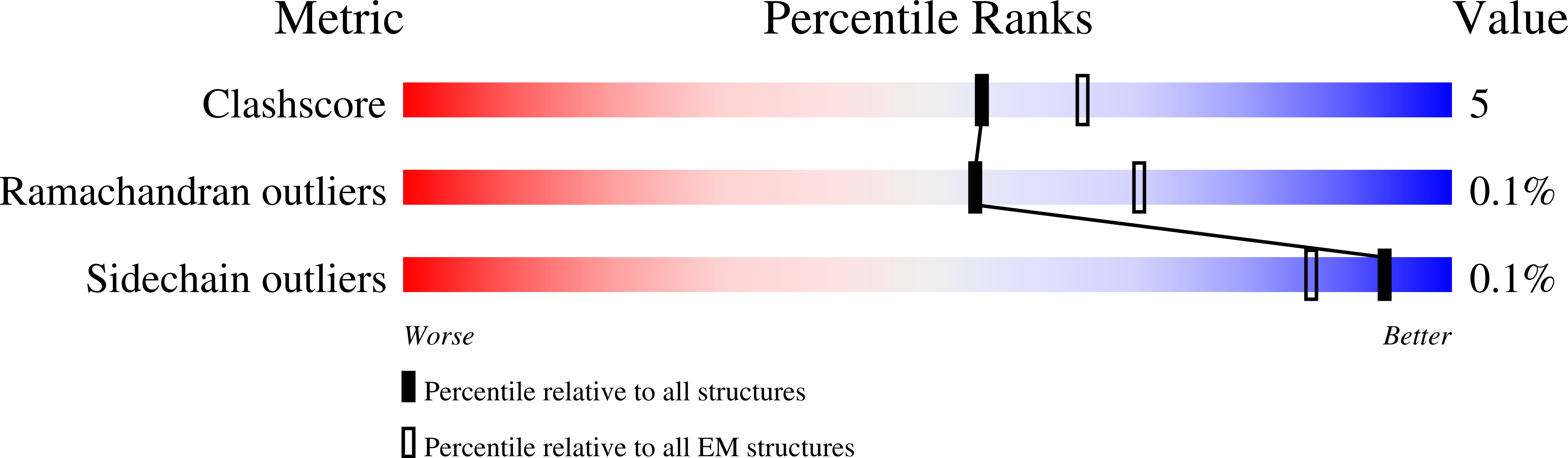Deposition Date
2017-06-08
Release Date
2017-07-12
Last Version Date
2024-10-23
Entry Detail
PDB ID:
5W3O
Keywords:
Title:
CryoEM structure of rhinovirus B14 in complex with C5 Fab (33 degrees Celsius, molar ratio 1:3, empty particle)
Biological Source:
Source Organism:
Mus musculus (Taxon ID: 10090)
Human rhinovirus 14 (Taxon ID: 12131)
Human rhinovirus 14 (Taxon ID: 12131)
Method Details:
Experimental Method:
Resolution:
3.01 Å
Aggregation State:
PARTICLE
Reconstruction Method:
SINGLE PARTICLE