Deposition Date
2017-05-02
Release Date
2017-08-02
Last Version Date
2024-10-09
Entry Detail
PDB ID:
5VOH
Keywords:
Title:
Crystal structure of engineered water-forming NADPH oxidase (TPNOX) bound to NADPH. The G159A, D177A, A178R, M179S, P184R mutant of LbNOX.
Biological Source:
Source Organism:
Lactobacillus brevis KB290 (Taxon ID: 1001583)
Host Organism:
Method Details:
Experimental Method:
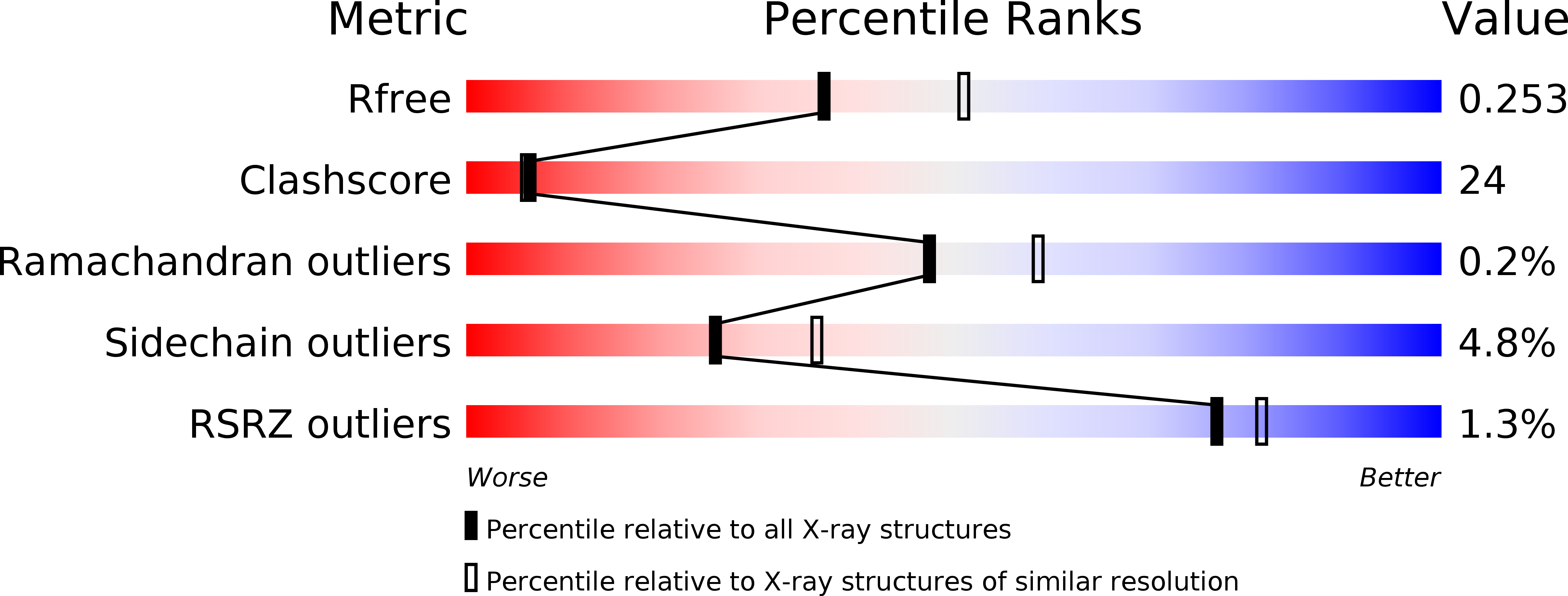
Resolution:
2.30 Å
R-Value Free:
0.25
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 1 21 1