Deposition Date
2017-03-20
Release Date
2017-10-11
Last Version Date
2023-11-15
Entry Detail
PDB ID:
5V7I
Keywords:
Title:
Crystal structure of homo sapiens serine hydroxymethyltransferase 2 (mitochondrial) (SHMT2), in complex with glycine, PLP and folate-competitive pyrazolopyran inhibitor: 6-amino-4-isopropyl-3-methyl-4-(3-(pyrrolidin-1-yl)-5-(trifluoromethyl)phenyl)-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile
Biological Source:
Source Organism(s):
Homo sapiens (Taxon ID: 9606)
Expression System(s):
Method Details:
Experimental Method:
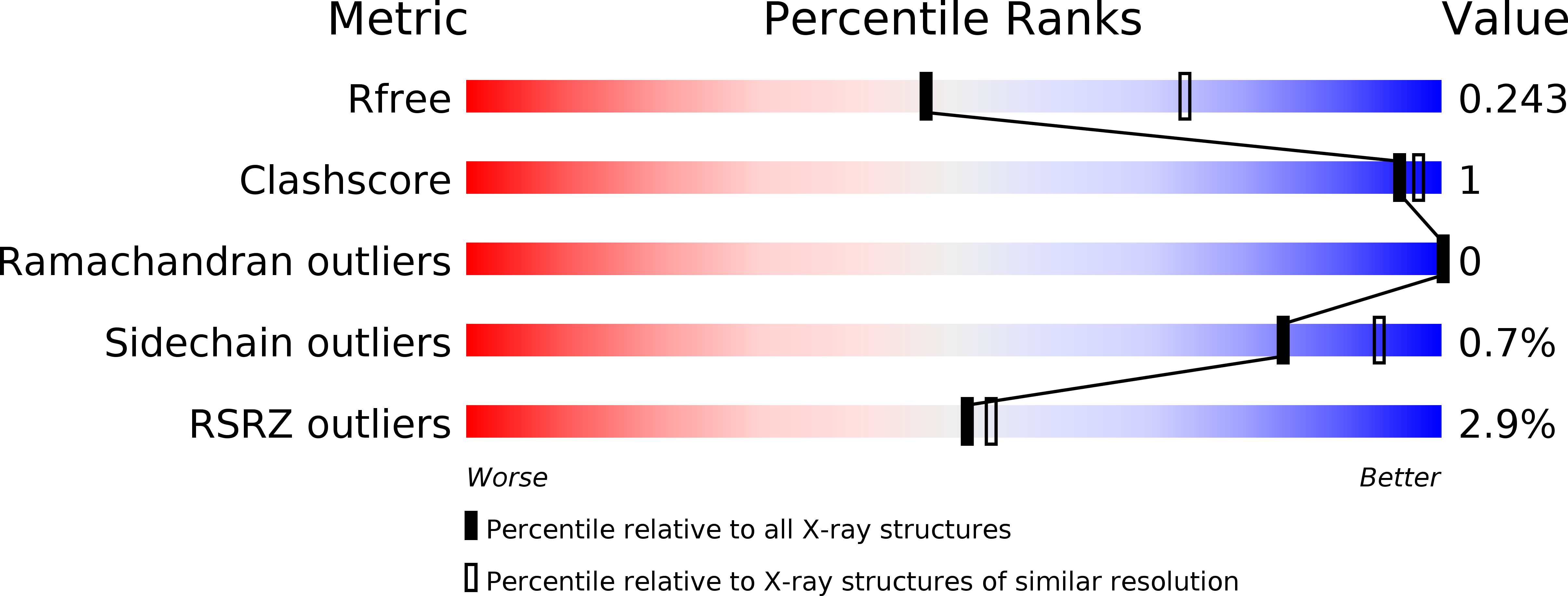
Resolution:
2.47 Å
R-Value Free:
0.23
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 65 2 2