Deposition Date
2017-03-15
Release Date
2017-06-28
Last Version Date
2025-04-02
Entry Detail
PDB ID:
5V65
Keywords:
Title:
Crystal structure of macrocycles containing Abeta 17-23 (LV(PHI)FAED) and Abeta 30-36 (AII(SAR)L(ORN)V)
Biological Source:
Source Organism(s):
synthetic construct (Taxon ID: 32630)
Method Details:
Experimental Method:
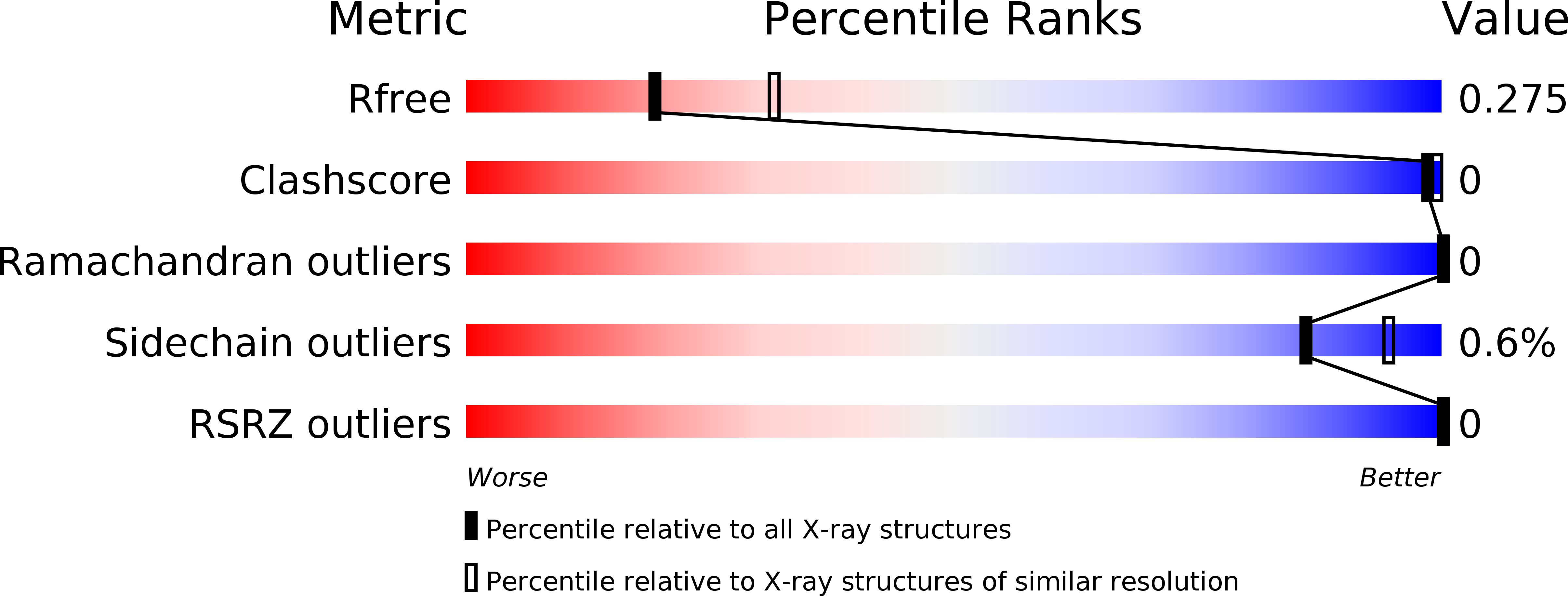
Resolution:
2.52 Å
R-Value Free:
0.28
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
H 3