Deposition Date
2017-01-22
Release Date
2018-02-21
Last Version Date
2024-03-06
Entry Detail
PDB ID:
5UKI
Keywords:
Title:
Mn2+ and Zn2+ requirements for the lariat debranching enzyme, Dbr1
Biological Source:
Source Organism:
Entamoeba histolytica (Taxon ID: 5759)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.80 Å
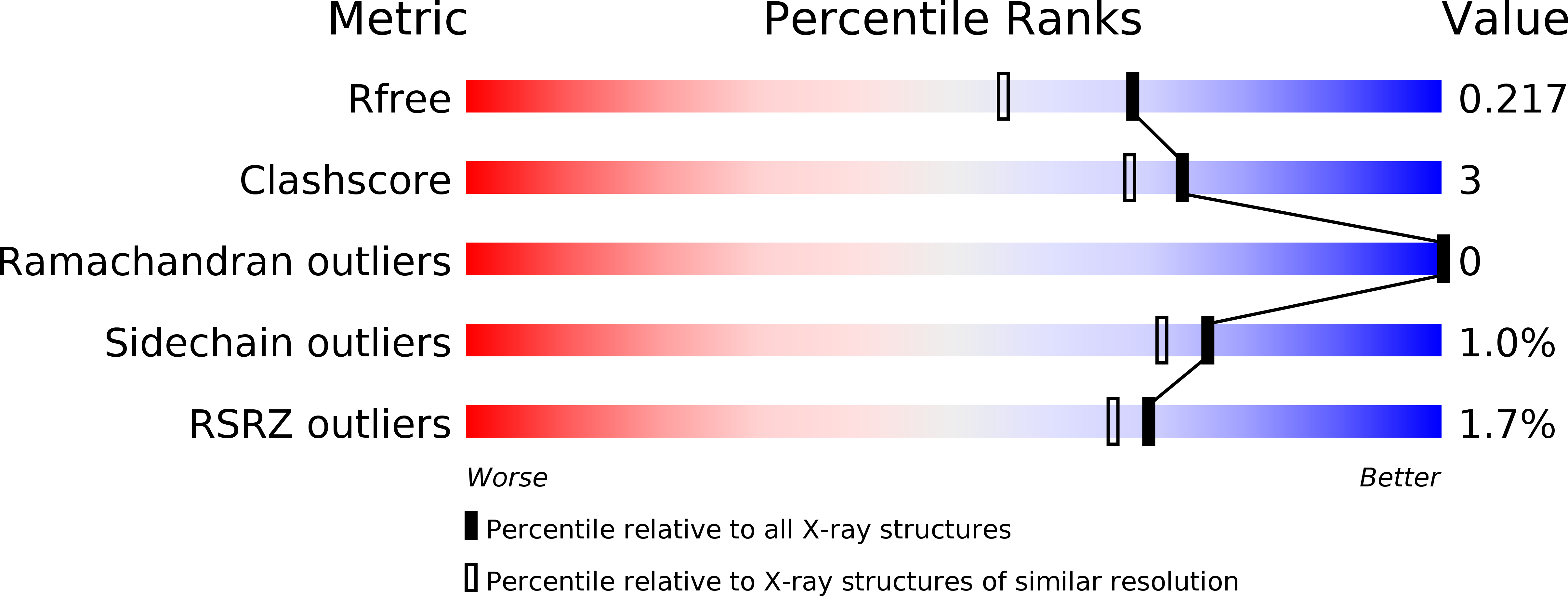
R-Value Free:
0.21
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 21 21 21