Deposition Date
2016-12-21
Release Date
2017-12-27
Last Version Date
2023-10-04
Entry Detail
PDB ID:
5UC1
Keywords:
Title:
Structural Analysis of Glucocorticoid Receptor beta Ligand Binding Domain Complexed with Glucocorticoid Antagonist RU-486: Implication of Helix 12 in Antagonism
Biological Source:
Source Organism:
Heterocephalus glaber (Taxon ID: 10181)
Host Organism:
Method Details:
Experimental Method:
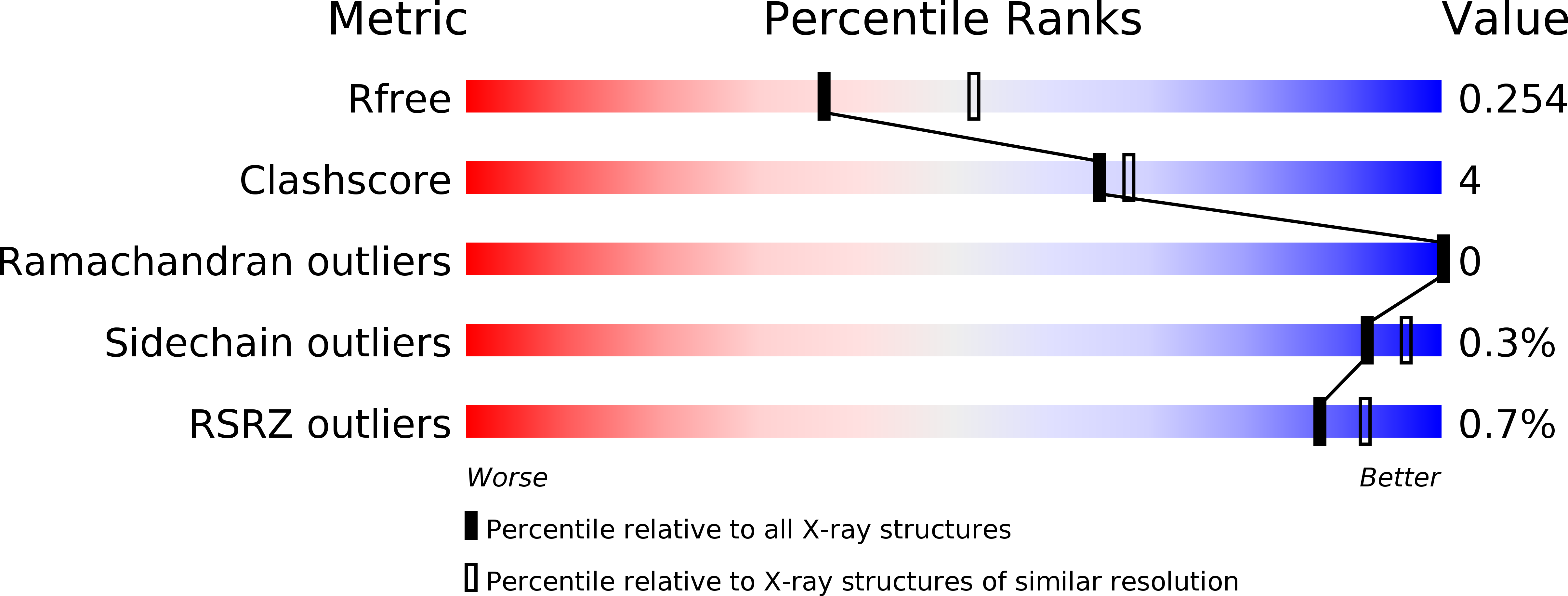
Resolution:
2.35 Å
R-Value Free:
0.25
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 61