Deposition Date
2016-12-19
Release Date
2017-01-25
Last Version Date
2024-10-16
Entry Detail
PDB ID:
5UAO
Keywords:
Title:
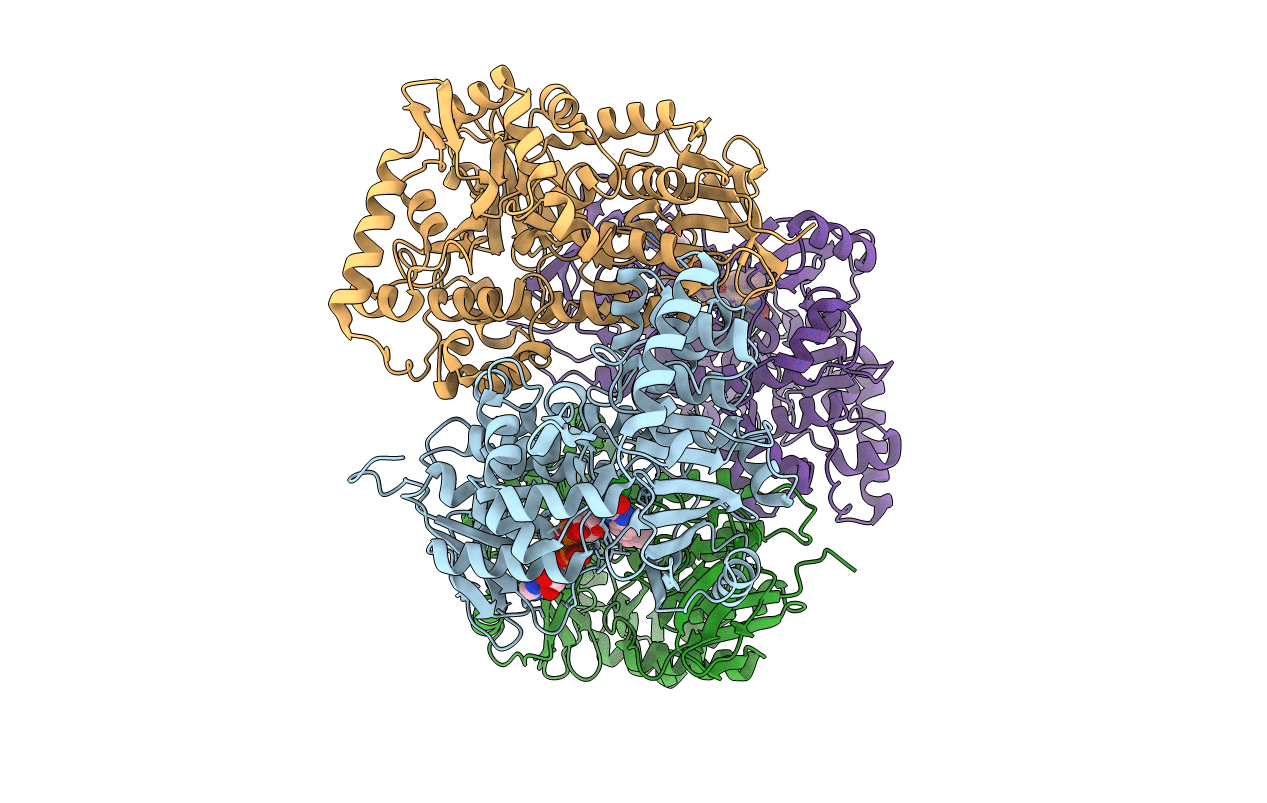
Crystal structure of MibH, a lathipeptide tryptophan 5-halogenase
Biological Source:
Source Organism:
Microbispora sp. ATCC PTA-5024 (Taxon ID: 316330)
Host Organism:
Method Details:
Experimental Method:
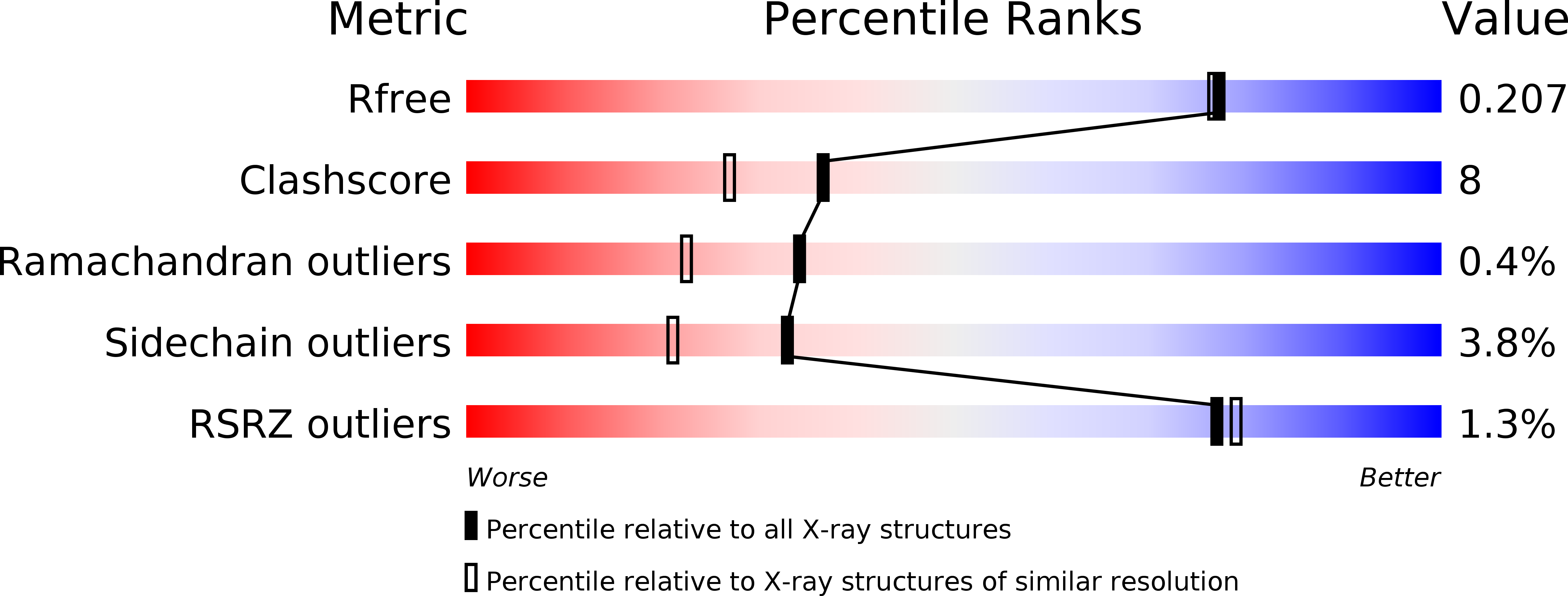
Resolution:
1.88 Å
R-Value Free:
0.20
R-Value Work:
0.15
R-Value Observed:
0.15
Space Group:
P 1