Deposition Date
2016-11-28
Release Date
2017-01-04
Last Version Date
2024-10-16
Entry Detail
PDB ID:
5U1L
Keywords:
Title:
Crystal structure of the ATP-gated P2X7 ion channel in the closed, apo state
Biological Source:
Source Organism(s):
Ailuropoda melanoleuca (Taxon ID: 9646)
Expression System(s):
Method Details:
Experimental Method:
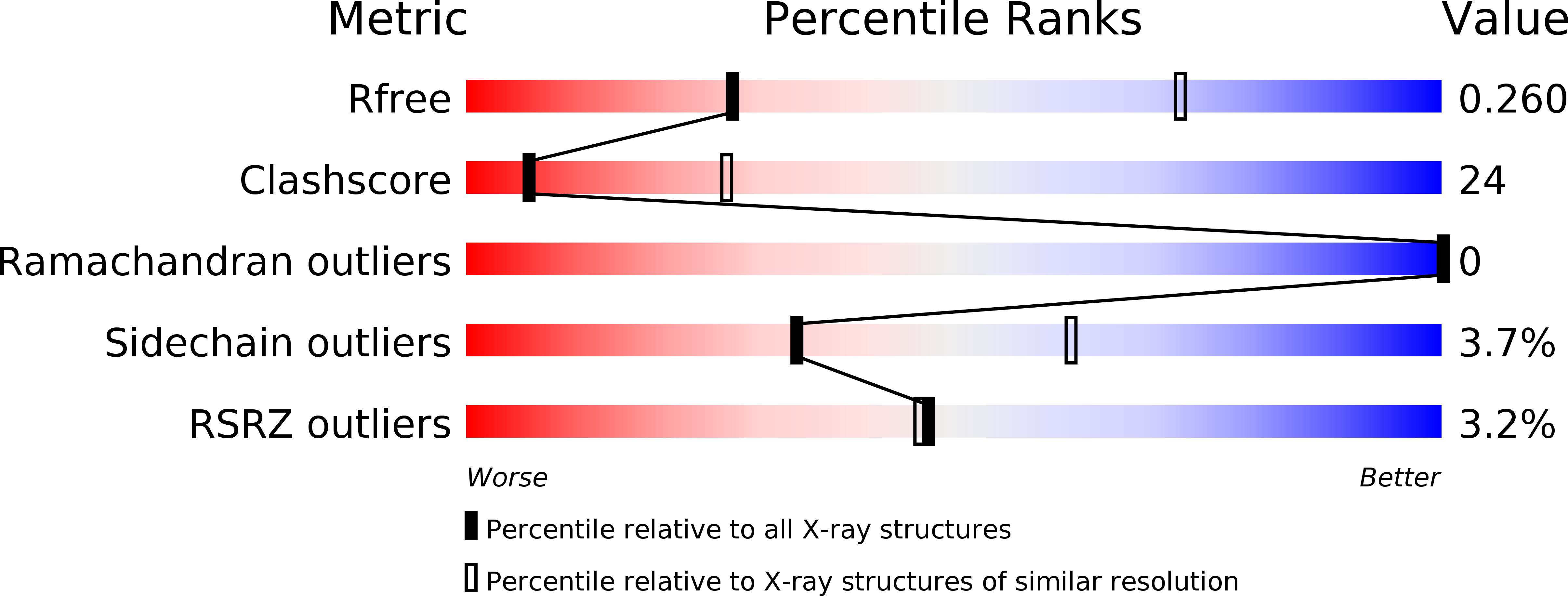
Resolution:
3.40 Å
R-Value Free:
0.26
R-Value Work:
0.24
R-Value Observed:
0.24
Space Group:
I 21 3