Deposition Date
2016-11-21
Release Date
2017-04-19
Last Version Date
2023-10-04
Entry Detail
PDB ID:
5TZG
Keywords:
Title:
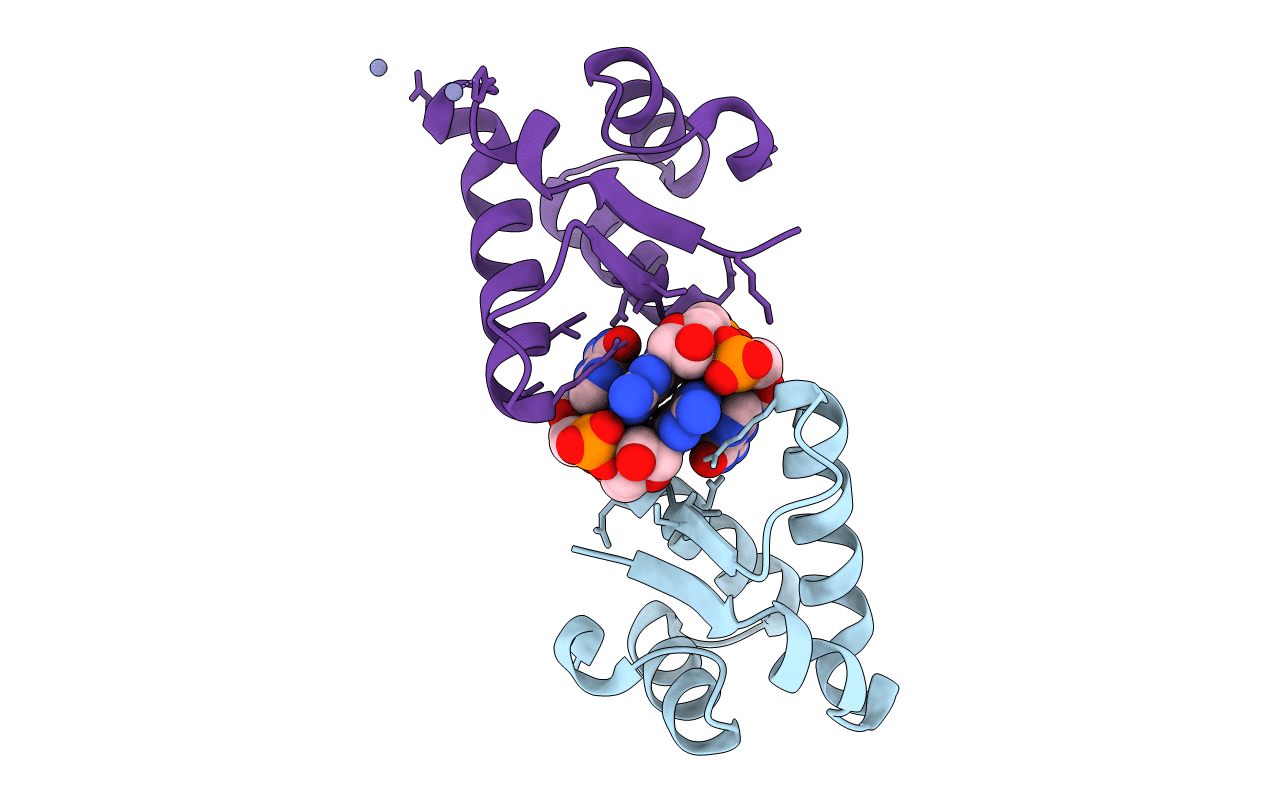
Structure of the BldD CTD(D116A)-(c-di-GMP)2, form 2
Biological Source:
Source Organism:
Streptomyces venezuelae (Taxon ID: 953739)
Host Organism:
Method Details:
Experimental Method:
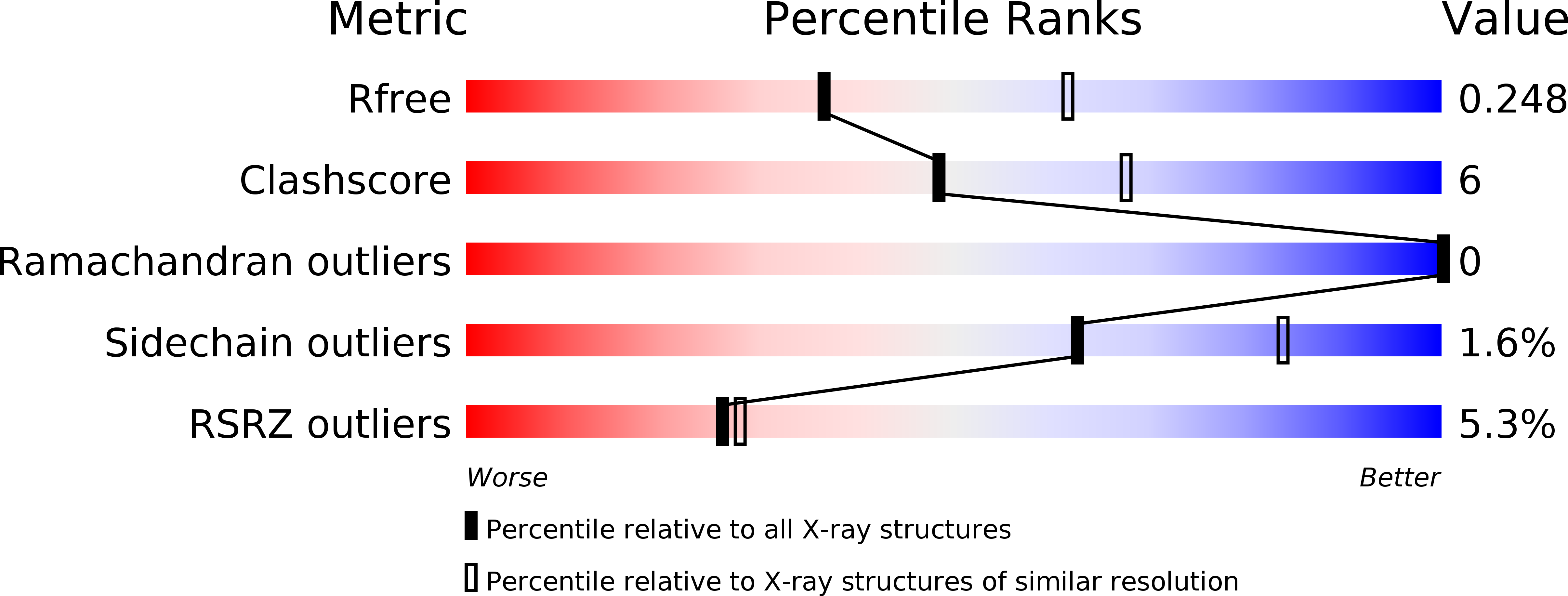
Resolution:
2.50 Å
R-Value Free:
0.24
R-Value Work:
0.19
R-Value Observed:
0.20
Space Group:
P 41 2 2