Deposition Date
2016-11-03
Release Date
2017-08-23
Last Version Date
2024-10-16
Entry Detail
PDB ID:
5TTH
Keywords:
Title:
Heterodimeric SpyCatcher/SpyTag-fused zebrafish TRAP1 in ATP/ADP-hybrid state
Biological Source:
Source Organism(s):
Danio rerio (Taxon ID: 7955)
Streptococcus pyogenes (Taxon ID: 1314)
Streptococcus pyogenes (Taxon ID: 1314)
Expression System(s):
Method Details:
Experimental Method:
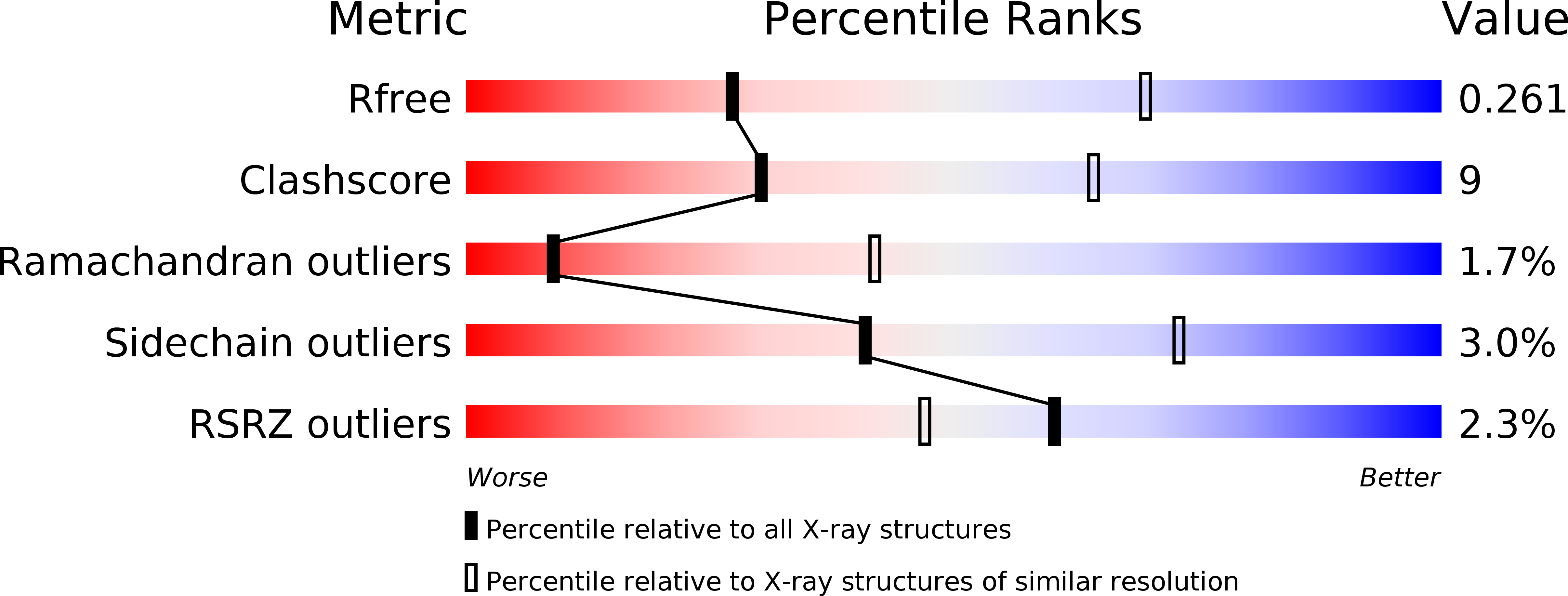
Resolution:
3.20 Å
R-Value Free:
0.26
R-Value Work:
0.22
R-Value Observed:
0.22
Space Group:
C 1 2 1